Search results
Search for "chiral auxiliaries" in Full Text gives 50 result(s) in Beilstein Journal of Organic Chemistry.
Selective and scalable oxygenation of heteroatoms using the elements of nature: air, water, and light
- Damiano Diprima,
- Hannes Gemoets,
- Stefano Bonciolini and
- Koen Van Aken
Beilstein J. Org. Chem. 2023, 19, 1146–1154, doi:10.3762/bjoc.19.82
- phosphine oxide, and selenides to selenoxides. Sulfoxide, phosphine oxide, and selenoxide-containing molecules have diverse applications in the pharmaceutical industry [10], as chiral auxiliaries or as ligands for asymmetric metal catalysis [11], and in materials such as polymers [12][13] and flame

Graphical Abstract

Scheme 1: Oxidation of heteroatoms.

Scheme 2: Graphical representation comparing A electrochemistry and B photoredox catalysis using a semiconduc...

Figure 1: Study of additives. A) Effect of the addition of 1 equiv of various acids and bases to the standard...

Scheme 3: Substrate scope with reaction times and isolated yields. 1 mmol (1 equiv) substrate was reacted in ...

Scheme 4: Setup used in the flow experiment for the triphenylphosphine oxidation.

Scheme 5: Proposed extra alternative pathway.
Photoredox catalysis harvesting multiple photon or electrochemical energies
- Mattia Lepori,
- Simon Schmid and
- Joshua P. Barham
Beilstein J. Org. Chem. 2023, 19, 1055–1145, doi:10.3762/bjoc.19.81

Graphical Abstract

Figure 1: Oxidative and reductive activations of organic compounds harvesting photoredox catalysis.

Figure 2: General catalytic cycles of radical ion conPET (left) and radical ion e-PRC (right).

Figure 3: “Beginner’s guide”: comparison between advantages, capacities, and prospectives of conPET and PEC.

Figure 4: A) conPET reductive dehalogenation of aryl halides with PDI. B) Reductive C–H arylation with pyrrol...

Figure 5: A) Chromoselective mono- and disubstitution or polybrominated pyrimidines with pyrroles. B) Sequent...

Figure 6: A) Synthesis of pyrrolo[1,2-a]quinolines. B) Synthesis of ullazines.

Figure 7: A) Reductive phosphorylation of aryl halides via conPET. B) Selected examples from the substrate sc...

Figure 8: A) Reductive dehalogenation of aryl halides via conPET and selected examples from the substrate sco...

Figure 9: A) Reductive C–H arylation of aryl halides via conPET (top) and selected examples from the substrat...

Figure 10: A) Reductive hydrodehalogenation of aryl halides with Mes-Acr-BF4. B) Selected examples from the su...

Figure 11: A) Reductive hydrodechlorination of aryl chlorides with 4-DPAIPN. B) Proposed formation of CO2•−. C...

Figure 12: A) Reductive conPET borylation with 3CzEPAIPN (top) and selected examples from the substrate scope ...

Figure 13: Scale-up of conPET phosphorylation with 3CzEPAIPN.

Figure 14: A) Borylation of 1d. B) Characteristics and structure of PC1 with green and red parts showing the l...

Figure 15: A) Reductive C–H arylation scope with polysulfide conPET (top) and selected examples from the subst...

Figure 16: Scale-up of A) C–H arylation and B) dehaloborylation with polysulfide photocatalysis in continuous-...

Figure 17: A) Formation of [Ir1]0 and [Ir2]0 upon PET between [Ir1]+ and Et3N. B) Mechanism of multi-photon ta...

Figure 18: A) Reductive hydrodehalogenation of aryl halides via multi-photon tandem photocatalysis. B) Selecte...

Figure 19: A) Carbonylative amidation of aryl halides in continuous flow. B) Selected examples from the substr...

Figure 20: A) General scheme for reductive (RQ) and oxidative quenching (OQ) protocols using [FeIII(btz)3](PF6)...

Figure 21: A) Carbonylative amidation of alkyl iodides with [IrIII(ppy)2(dtbbpy)]PF6. B) Selected examples fro...

Figure 22: A) Carboxylative C–N bond cleavage in cyclic amines. B) Selected examples from the substrate scope....

Figure 23: A) Formal reduction of alkenes to alkanes via transfer hydrogenation. B) Selected examples from the...

Figure 24: A) Birch-type reduction of benzenes with PMP-BPI. B) Selected examples from the substrate scope (sc...

Figure 25: Proposed mechanism of the OH− mediated conPET Birch-type reduction of benzene via generation of sol...

Figure 26: Reductive detosylation of N-tosylated amides with Mes-Acr-BF4. B) Selected examples from the substr...

Figure 27: A) Reductive detosylation of N-tosyl amides by dual PRC. B) Selected examples from the substrate sc...

Figure 28: A) Mechanism of the dual PRC based on PET between [Cu(dap)2]+ and DCA. B) Mechanism of the dual PRC...

Figure 29: A) N–O bond cleavage in Weinreb amides with anthracene. B) N–O bond cleavage in Weinreb amides rely...

Figure 30: A) Pentafluorosulfanylation and fluoride elimination. B) Mechanism of the pentafluorosulfanylation ...

Figure 31: A) α-Alkoxypentafluorosulfanylation (top) and selected examples from the substrate scope (bottom). ...

Figure 32: A) Oxidative amination of arenes with azoles catalyzed by N-Ph PTZ. B) Selected examples from the s...

Figure 33: A) C(sp3)–H bond activation by HAT via chloride oxidation by *N-Ph PTZ•+. B) Proposed mechanism for...

Figure 34: A) Recycling e-PRC C–H azolation of electron-rich arenes with pyrazoles using Mes-Acr+ as a photoca...

Figure 35: A) Radical ion e-PRC direct oxidation of unactivated arenes using TAC+ as an electro-activated phot...

Figure 36: A) Radical ion e-PRC direct oxidation of unactivated arenes using TPA as an electro-activated photo...

Figure 37: Proposed mechanism (top) and mode of preassembly (bottom).

Figure 38: A) Possible preassemblies of reactive (left) vs unreactive (right) arenes. B) Calculated spin densi...

Figure 39: A) Recycling e-PRC C(sp2 )–H acetoxylation of arenes using DDQ as a photocatalyst. B) Proposed cata...

Figure 40: Gram scale hydroxylation of benzene in a recirculated flow setup.

Figure 41: A) Radical ion e-PRC vicinal diamination of alkylarenes using TAC+ as an electro-activated photocat...

Figure 42: A) Sequential oxygenation of multiple adjacent C–H bonds under radical ion e-PRC using TAC+ as an e...

Figure 43: A) Enantioselective recycling e-PRC cyanation of benzylic C–H bonds using ADQS as photocatalyst. B)...

Figure 44: Proposed tandem mechanism by Xu and co-workers.

Figure 45: A) Enantioselective recycling e-PRC decarboxylative cyanation using Cu(acac)2, Ce(OTf)3 and a box l...

Figure 46: A) Enantioselective recycling e-PRC benzylic cyanation using Cu(MeCN)4BF4, box ligand and anthraqui...

Figure 47: A) Radical ion e-PRC acetoxyhydroxylation of aryl olefins using TAC+ as an electro-activated photoc...

Figure 48: Selected examples from the substrate scope.

Figure 49: Photoelectrochemical acetoxyhydroxylation in a recirculated flow setup.

Figure 50: A) Radical ion e-PRC aminooxygenation of aryl olefins using TAC+ as an electro-activated photocatal...

Figure 51: A) Recycling e-PRC C–H alkylation of heteroarenes with organic trifluoroborates using Mes-Acr+ as p...

Figure 52: A) Recycling e-PRC decarboxylative C–H alkylation of heteroarenes using CeCl3·7H2O as catalyst. B) ...

Figure 53: A) Recycling e-PRC decarboxylative C–H alkylation of heteroarenes using Fe(NH4)2(SO4)2·6H2O as cata...

Figure 54: A) Recycling e-PRC C–H alkylation of heteroarenes with alkyl oxalates and 4CzIPN as photocatalyst. ...

Figure 55: A) Recycling e-PRC decarboxylative C–H carbamoylation of heteroarenes using 4CzIPN as photocatalyst...

Figure 56: A) Photoelectrochemical HAT-mediated hydrocarbon activation via the chlorine radical. B) Proposed m...

Figure 57: A) Selected examples from the substrate scope. B) Gram and decagram scale semi-continuous flow PEC ...

Figure 58: A) Photoelectrochemical HAT-mediated dehydrogenative coupling of benzothiazoles with aliphatic C–H ...

Figure 59: A) Photoelectrochemical HAT activation of ethers using electro-activated TAC+ as photocatalyst. B) ...

Figure 60: Selected examples from the substrate scope.

Figure 61: A) Photoelectrochemical HAT-mediated synthesis of alkylated benzimidazo-fused isoquinolinones using...

Figure 62: A) Decoupled photoelectrochemical cerium-catalyzed oxydichlorination of alkynes using CeCl3 as cata...

Figure 63: Proposed decoupled photoelectrochemical mechanism.

Figure 64: A) Decoupled photoelectrochemical ring-opening bromination of tertiary cycloalkanols using MgBr2 as...

Figure 65: A) Recycling e-PRC ring-opening functionalization of cycloalkanols using CeCl3 as catalyst. B) Prop...

Figure 66: Selected examples from the substrate scope of the PEC ring-opening functionalization.

Figure 67: A) Radical ion e-PRC reduction of chloro- and bromoarenes using DCA as catalyst and various accepto...

Figure 68: A) Screening of different phthalimide derivatives as catalyst for the e-PRC reduction of aryl halid...

Figure 69: Screening of different organic catalysts for the e-PRC reduction of trialkylanilium salts.

Figure 70: A) e-PRC reduction of phosphonated phenols and anilinium salts. B) Selected examples from the subst...

Figure 71: A) ConPET and e-PRC reduction of 4-bromobenzonitrile using a naphthalene diimide (NDI) precatalyst ...

Figure 72: A) Radical ion e-PRC reduction of phosphinated aliphatic alcohols with n-BuO-NpMI as catalyst. B) C...

Figure 73: Selected examples from the substrate scope.

Figure 74: A) Recycling e-PRC reductive dimerization of benzylic chlorides using a [Cu2] catalyst. B) Proposed...

Figure 75: A) Decoupled photoelectrochemical C–H alkylation of heteroarenes through deamination of Katritzky s...

Figure 76: Proposed mechanism by Chen and co-workers.
A new oxidatively stable ligand for the chiral functionalization of amino acids in Ni(II)–Schiff base complexes
- Alena V. Dmitrieva,
- Oleg A. Levitskiy,
- Yuri K. Grishin and
- Tatiana V. Magdesieva
Beilstein J. Org. Chem. 2023, 19, 566–574, doi:10.3762/bjoc.19.41

- complex. Solubility of the t-Bu-containing ligand and its Schiff base complexes is increased, facilitating scaling-up the reaction procedure and isolation of the functionalized amino acid. Keywords: asymmetric synthesis; chiral auxiliaries; cysteine derivatives; Ni–Schiff base complexes; voltammetry
- employing chiral auxiliaries [4][5] and asymmetric phase-transfer catalysis [6][7]. The former approach is commonly based on the application of chiral derivatives of glycine containing structurally diverse chiral auxiliaries, both cyclic [8][9][10][11] and acyclic [12][13]. Transition-metal complexes
- )) and includes a chiral auxiliary, an amino acid, and a bifunctional linker capable to arrange the components in the Schiff base complex. Such templates provide a significant C–H acidity at the α-amino acid carbon and a possibility for recycling of the chiral auxiliaries (for reviews see [5][14][15][16

Graphical Abstract

Scheme 1: Selected examples of the chiral ligands used for synthesis of the Ni(II)–Schiff base complexes.

Scheme 2: Synthesis of the chiral ligand L7 and its Ni(II) complexes with glycine, serine, dehydroalanine, an...

Figure 1: Fragment of the NOESY spectrum of the ʟ-(oBrCysNi)L7 complex indicating the correlation between the...

Figure 2: Low-gradient isosurfaces with low densities (blue color of the isosurface corresponds to the hydrog...

Figure 3: Saturated solutions of (GlyNi)L1 (left) and (GlyNi)L7 (right) in diethyl ether.

Figure 4: The CV curves observed for (GlyNi)L7 and (ΔAlaNi)L7 in the anodic and cathodic regions (Pt, CH3CN, ...
An efficient metal-free and catalyst-free C–S/C–O bond-formation strategy: synthesis of pyrazole-conjugated thioamides and amides
- Shubham Sharma,
- Dharmender Singh,
- Sunit Kumar,
- Vaishali,
- Rahul Jamra,
- Naveen Banyal,
- Deepika,
- Chandi C. Malakar and
- Virender Singh
Beilstein J. Org. Chem. 2023, 19, 231–244, doi:10.3762/bjoc.19.22
- ]. Substituted pyrazoles are also of considerable interest because of their synthetic utility as chiral auxiliaries [32], synthetic reagents in multicomponent reactions [33][34], and guanylating agents [35]. The installation of a thioamide functionality has attracted an immense attention in medicinal chemistry

Graphical Abstract

Figure 1: Representative drug molecules based on pyrazole, thioamide, and amide derivatives.

Figure 2: Previous and present findings for the synthesis of thioamide derivatives.

Scheme 1: Synthesis of pyrazole C-3-tethered thioamides.

Scheme 2: Synthesis of pyrazole C-4-tethered thioamides.

Scheme 3: Metal- and catalyst-free preparation of pyrazole C-5-linked thioamide conjugates.

Scheme 4: Synthesis of 4-iodopyrazole C-3-tethered thioamides.

Scheme 5: Gram-scale scope of the current protocol.

Scheme 6: Control experiment.

Scheme 7: H2O2-mediated synthesis of pyrazole-pyridine conjugates with amide tethers.

Scheme 8: Synthesis of pyrazole-pyridine conjugates 9F and 10F having amide tethers.

Scheme 9: A tentative mechanism for the formation of pyrazole conjugates with thioamide and amide linkage.
Electrochemical formal homocoupling of sec-alcohols
- Kosuke Yamamoto,
- Kazuhisa Arita,
- Masashi Shiota,
- Masami Kuriyama and
- Osamu Onomura
Beilstein J. Org. Chem. 2022, 18, 1062–1069, doi:10.3762/bjoc.18.108
- ]. Such scaffolds are widely utilized as versatile building blocks in the synthesis of biologically active compounds [3][4][5][6][7], chiral auxiliaries [8][9], and chiral ligands [10][11][12][13]. Traditional pinacol coupling reactions are performed with a stoichiometric or even excess amount of low

Graphical Abstract

Scheme 1: Strategies for the synthesis of vic-1,2-diols.

Scheme 2: Substrate scope. Reaction conditions: 1 (1.0 mmol), Et4NBr (0.1 equiv), imidazole (0.05 equiv), MeC...

Scheme 3: Investigation of cross-coupling reaction.

Scheme 4: Large-scale experiment.

Scheme 5: Control experiments. aDetermined by 1H NMR using 1,3,5-trimethoxybenzene as an internal standard. b...

Scheme 6: Proposed mechanism.
Iron-catalyzed domino coupling reactions of π-systems
- Austin Pounder and
- William Tam
Beilstein J. Org. Chem. 2021, 17, 2848–2893, doi:10.3762/bjoc.17.196

Graphical Abstract

Figure 1: Price comparison among iron and other transition metals used in catalysis.

Scheme 1: Typical modes of C–C bond formation.

Scheme 2: The components of an iron-catalyzed domino reaction.

Scheme 3: Iron-catalyzed tandem cyclization and cross-coupling reactions of iodoalkanes 1 with aryl Grignard ...

Scheme 4: Three component iron-catalyzed dicarbofunctionalization of vinyl cyclopropanes 14.

Scheme 5: Three-component iron-catalyzed dicarbofunctionalization of alkenes 21.

Scheme 6: Double carbomagnesiation of internal alkynes 31 with alkyl Grignard reagents 32.

Scheme 7: Iron-catalyzed cycloisomerization/cross-coupling of enyne derivatives 35 with alkyl Grignard reagen...

Scheme 8: Iron-catalyzed spirocyclization/cross-coupling cascade.

Scheme 9: Iron-catalyzed alkenylboration of alkenes 50.

Scheme 10: N-Alkyl–N-aryl acrylamide 60 CDC cyclization with C(sp3)–H bonds adjacent to a heteroatom.

Scheme 11: 1,2-Carboacylation of activated alkenes 60 with aldehydes 65 and alcohols 67.

Scheme 12: Iron-catalyzed dicarbonylation of activated alkenes 68 with alcohols 67.

Scheme 13: Iron-catalyzed cyanoalkylation/radical dearomatization of acrylamides 75.

Scheme 14: Synergistic photoredox/iron-catalyzed 1,2-dialkylation of alkenes 82 with common alkanes 83 and 1,3...

Scheme 15: Iron-catalyzed oxidative coupling/cyclization of phenol derivatives 86 and alkenes 87.

Scheme 16: Iron-catalyzed carbosulfonylation of activated alkenes 60.

Scheme 17: Iron-catalyzed oxidative spirocyclization of N-arylpropiolamides 91 with silanes 92 and tert-butyl ...

Scheme 18: Iron-catalyzed free radical cascade difunctionalization of unsaturated benzamides 94 with silanes 92...

Scheme 19: Iron-catalyzed cyclization of olefinic dicarbonyl compounds 97 and 100 with C(sp3)–H bonds.

Scheme 20: Radical difunctionalization of o-vinylanilides 102 with ketones and esters 103.

Scheme 21: Dehydrogenative 1,2-carboamination of alkenes 82 with alkyl nitriles 76 and amines 105.

Scheme 22: Iron-catalyzed intermolecular 1,2-difunctionalization of conjugated alkenes 107 with silanes 92 and...

Scheme 23: Four-component radical difunctionalization of chemically distinct alkenes 114/115 with aldehydes 65...

Scheme 24: Iron-catalyzed carbocarbonylation of activated alkenes 60 with carbazates 117.

Scheme 25: Iron-catalyzed radical 6-endo cyclization of dienes 119 with carbazates 117.

Scheme 26: Iron-catalyzed decarboxylative synthesis of functionalized oxindoles 130 with tert-butyl peresters ...

Scheme 27: Iron‑catalyzed decarboxylative alkylation/cyclization of cinnamamides 131/134.

Scheme 28: Iron-catalyzed carbochloromethylation of activated alkenes 60.

Scheme 29: Iron-catalyzed trifluoromethylation of dienes 142.

Scheme 30: Iron-catalyzed, silver-mediated arylalkylation of conjugated alkenes 115.

Scheme 31: Iron-catalyzed three-component carboazidation of conjugated alkenes 115 with alkanes 101/139b and t...

Scheme 32: Iron-catalyzed carboazidation of alkenes 82 and alkynes 160 with iodoalkanes 20 and trimethylsilyl ...

Scheme 33: Iron-catalyzed asymmetric carboazidation of styrene derivatives 115.

Scheme 34: Iron-catalyzed carboamination of conjugated alkenes 115 with alkyl diacyl peroxides 163 and acetoni...

Scheme 35: Iron-catalyzed carboamination using oxime esters 165 and arenes 166.

Scheme 36: Iron-catalyzed iminyl radical-triggered [5 + 2] and [5 + 1] annulation reactions with oxime esters ...

Scheme 37: Iron-catalyzed decarboxylative alkyl etherification of alkenes 108 with alcohols 67 and aliphatic a...

Scheme 38: Iron-catalyzed inter-/intramolecular alkylative cyclization of carboxylic acid and alcohol-tethered...

Scheme 39: Iron-catalyzed intermolecular trifluoromethyl-acyloxylation of styrene derivatives 115.

Scheme 40: Iron-catalyzed carboiodination of terminal alkenes and alkynes 180.

Scheme 41: Copper/iron-cocatalyzed cascade perfluoroalkylation/cyclization of 1,6-enynes 183/185.

Scheme 42: Iron-catalyzed stereoselective carbosilylation of internal alkynes 187.

Scheme 43: Synergistic photoredox/iron catalyzed difluoroalkylation–thiolation of alkenes 82.

Scheme 44: Iron-catalyzed three-component aminoazidation of alkenes 82.

Scheme 45: Iron-catalyzed intra-/intermolecular aminoazidation of alkenes 194.

Scheme 46: Stereoselective iron-catalyzed oxyazidation of enamides 196 using hypervalent iodine reagents 197.

Scheme 47: Iron-catalyzed aminooxygenation for the synthesis of unprotected amino alcohols 200.

Scheme 48: Iron-catalyzed intramolecular aminofluorination of alkenes 209.

Scheme 49: Iron-catalyzed intramolecular aminochlorination and aminobromination of alkenes 209.

Scheme 50: Iron-catalyzed intermolecular aminofluorination of alkenes 82.

Scheme 51: Iron-catalyzed aminochlorination of alkenes 82.

Scheme 52: Iron-catalyzed phosphinoylazidation of alkenes 108.

Scheme 53: Synergistic photoredox/iron-catalyzed three-component aminoselenation of trisubstituted alkenes 82.
Synthetic strategies toward 1,3-oxathiolane nucleoside analogues
- Umesh P. Aher,
- Dhananjai Srivastava,
- Girij P. Singh and
- Jayashree B. S
Beilstein J. Org. Chem. 2021, 17, 2680–2715, doi:10.3762/bjoc.17.182
- -oxathiolane nucleosides via resolution methods. The chemical as well as enzymatic procedures are reviewed and segregated in this review for effective synthesis of 1,3-oxathiolane nucleoside analogues. Keywords: chiral auxiliaries; enzymes; Lewis acids; N-glycosylation; 1,3-oxathiolane sugar and nucleosides
- introduction on important classical approaches and older yet creative methods to provide the reader with a historical context. For comparison, this will be followed by a discussion of more modern techniques, including chiral auxiliaries for neighboring group participation and transition metal-catalyzed

Graphical Abstract

Figure 1: Representative modified 1,3-oxathiolane nucleoside analogues.

Figure 2: Mechanism of antiviral action of 1,3-oxathiolane nucleosides, 3TC (1) and FTC (2), as chain termina...

Figure 3: Synthetic strategies for the construction of the 1,3-oxathiolane sugar ring.

Scheme 1: Synthesis of 4 from benzoyloxyacetaldehyde (3a) and 2-mercapto-substituted dimethyl acetal 3na.

Scheme 2: Synthesis of 8 from protected glycolic aldehyde 3b and 2-mercaptoacetic acid (3o).

Scheme 3: Synthesis of 20 from ᴅ-mannose (3c).

Scheme 4: Synthesis of 20 from 1,6-thioanhydro-ᴅ-galactose (3d).

Scheme 5: Synthesis of 8 from 2-(tert-butyldiphenylsilyloxy)methyl-5-oxo-1,2-oxathiolane (3m).

Scheme 6: Synthesis of 20a from ʟ-gulose derivative 3f.

Scheme 7: Synthesis of 31 from (+)-thiolactic acid 3p and 2-benzoyloxyacetaldehyde (3a).

Scheme 8: Synthesis of 35a from 1,4-dithiane-2,5-diol (3q) and glyoxylic acid (3g) hydrate.

Scheme 9: Synthetic routes toward 41 through Pummerer reaction from methyl 2-mercaptoacetate (3j) and bromoac...

Scheme 10: Strategy for the synthesis of 2,5-substituted 1,3-oxathiolane 41a using 4-nitrobenzyl glyoxylate an...

Scheme 11: Synthesis of 44 by a resolution method using Mucor miehei lipase.

Scheme 12: Synthesis of 45 from benzoyloxyacetaldehyde (3a) and 2-mercaptoacetaldehyde bis(2-methoxyethyl) ace...

Scheme 13: Synthesis of 46 from 2-mercaptoacetaldehyde bis(2-methoxyethyl) acetal (3nc) and diethyl 3-phosphon...

Scheme 14: Synthesis of 48 from 1,3-dihydroxyacetone dimer 3l.

Scheme 15: Approach toward 52 from protected alkene 3rb and lactic acid derivative 51 developed by Snead et al....

Scheme 16: Recent approach toward 56a developed by Kashinath et al.

Scheme 17: Synthesis of 56a from ʟ-menthyl glyoxylate (3h) hydrate by DKR.

Scheme 18: Possible mechanism with catalytic TEA for rapid interconversion of isomers.

Scheme 19: Synthesis of 35a by a classical resolution method through norephedrine salt 58 formation.

Scheme 20: Synthesis of 63 via [1,2]-Brook rearrangement from silyl glyoxylate 61 and thiol 3nb.

Scheme 21: Combined use of STS and CAL-B as catalysts to synthesize an enantiopure oxathiolane precursor 65.

Scheme 22: Synthesis of 1 and 1a from glycolaldehyde dimer 64 and 1,4-dithiane-2,5-diol (3q) using STS and CAL...

Scheme 23: Synthesis of 68 by using Klebsiella oxytoca.

Scheme 24: Synthesis of 71 and 72 using Trichosporon taibachii lipase and kinetic resolution.

Scheme 25: Synthesis of 1,3-oxathiolan-5-ones 77 and 78 via dynamic covalent kinetic resolution.

Figure 4: Pathway for glycosidic bond formation.

Scheme 26: First synthesis of (±)-BCH-189 (1c) by Belleau et al.

Scheme 27: Enantioselective synthesis of 3TC (1).

Scheme 28: Synthesis of cis-diastereomer 3TC (1) from oxathiolane propionate 44.

Scheme 29: Synthesis of (±)-BCH-189 (1c) via SnCl4-mediated N-glycosylation of 8.

Scheme 30: Synthesis of (+)-BCH-189 (1a) via TMSOTf-mediated N-glycosylation of 20.

Scheme 31: Synthesis of 3TC (1) from oxathiolane precursor 20a.

Scheme 32: Synthesis of 83 via N-glycosylation of 20 with pyrimidine bases.

Scheme 33: Synthesis of 85 via N-glycosylation of 20 with purine bases.

Scheme 34: Synthesis of 86 and 87 via N-glycosylation using TMSOTf and pyrimidines.

Scheme 35: Synthesis of 90 and 91 via N-glycosylation using TMSOTf and purines.

Scheme 36: Synthesis of 3TC (1) via TMSI-mediated N-glycosylation.

Scheme 37: Stereoselective N-glycosylation for the synthesis of 1 by anchimeric assistance of a chiral auxilia...

Scheme 38: Whitehead and co-workers’ approach for the synthesis of 1 via direct N-glycosylation without an act...

Scheme 39: ZrCl4-mediated stereoselective N-glycosylation.

Scheme 40: Plausible reaction mechanism for stereoselective N-glycosylation using ZrCl4.

Scheme 41: Synthesis of enantiomerically pure oxathiolane nucleosides 1 and 2.

Scheme 42: Synthesis of tetrazole analogues of 1,3-oxathiolane nucleosides 97.

Scheme 43: Synthetic approach toward 99 from 1,3-oxathiolane 45 by Camplo et al.

Scheme 44: Synthesis of 100 from oxathiolane phosphonate analogue 46.

Scheme 45: Synthetic approach toward 102 and the corresponding cyclic thianucleoside monophosphate 102a by Cha...

Scheme 46: Synthesis of emtricitabine (2) from 1,4-dithiane-2,5-diol (3q) and glyoxylic acid (3g).

Scheme 47: Synthesis of 1 and 2, respectively, from 56a–d using iodine-mediated N-glycosylation.

Scheme 48: Plausible mechanism for silane- and I2-mediated N-glycosylation.

Scheme 49: Pyridinium triflate-mediated N-glycosylation of 35a.

Scheme 50: Possible pathway for stereoselective N-glycosylation via in situ chelation with a metal ligand.

Scheme 51: Synthesis of novel 1,3-oxathiolane nucleoside 108 from oxathiolane precursor 8 and 3-benzyloxy-2-me...

Scheme 52: Synthesis of 110 using T-705 as a nucleobase and 1,3-oxathiolane derivative 8 via N-glycosylation.

Scheme 53: Synthesis of 1 using an asymmetric leaving group and N-glycosylation with bromine and mesitylene.

Scheme 54: Cytidine deaminase for enzymatic separation of 1c.

Scheme 55: Enzymatic resolution of the monophosphate derivative 116 for the synthesis of (−)-BCH-189 (1) and (...

Scheme 56: Enantioselective resolution by PLE-mediated hydrolysis to obtain FTC (2).

Scheme 57: (+)-Menthyl chloroformate as a resolving agent to separate a racemic mixture 120.

Scheme 58: Separation of racemic mixture 1c by cocrystal 123 formation with (S)-(−)-BINOL.
Recent advances in organocatalytic asymmetric aza-Michael reactions of amines and amides
- Pratibha Sharma,
- Raakhi Gupta and
- Raj K. Bansal
Beilstein J. Org. Chem. 2021, 17, 2585–2610, doi:10.3762/bjoc.17.173
- as an important synthetic strategy for the preparation of a large variety of β-amino carbonyl and similar motifs which are present in many bioactive natural products [8][9], antibiotics [10][11][12] and chiral auxiliaries [13][14][15]. However, the reaction of many nitrogen-nucleophiles, such as

Graphical Abstract

Scheme 1: Asymmetric aza-Michael addition catalyzed by cinchona alkaloid derivatives.

Scheme 2: Intramolecular 6-exo-trig aza-Michael addition reaction.

Scheme 3: Asymmetric aza-Michael/Michael addition cascade reaction of 2-nitrobenzofurans and 2-nitrobenzothio...

Scheme 4: Asymmetric aza-Michael addition of para-dienone imide to benzylamine.

Scheme 5: Asymmetric synthesis of chiral N-functionalized heteroarenes.
On the application of 3d metals for C–H activation toward bioactive compounds: The key step for the synthesis of silver bullets
- Renato L. Carvalho,
- Amanda S. de Miranda,
- Mateus P. Nunes,
- Roberto S. Gomes,
- Guilherme A. M. Jardim and
- Eufrânio N. da Silva Júnior
Beilstein J. Org. Chem. 2021, 17, 1849–1938, doi:10.3762/bjoc.17.126

- compounds and biaryl chiral auxiliaries. Also, the oxidative coupling of phenolic substrates has been reported to be mediated by vanadium complexes such as VCl4, VOCl3, and VOF3, among others. For instance, an intramolecular coupling of phenolic moieties using VOF3 has been reported as a final step in the

Graphical Abstract

Scheme 1: Schematic overview of transition metals studied in C–H activation processes.

Scheme 2: (A) Known biological activities related to benzimidazole-based compounds; (B and C) an example of a...

Scheme 3: (A) Known biological activities related to quinoline-based compounds; (B and C) an example of a sca...

Scheme 4: (A) Known biological activities related to sulfur-containing compounds; (B and C) an example of a s...

Scheme 5: (A) Known biological activities related to aminoindane derivatives; (B and C) an example of a scand...

Scheme 6: (A) Known biological activities related to norbornane derivatives; (B and C) an example of a scandi...

Scheme 7: (A) Known biological activities related to aniline derivatives; (B and C) an example of a titanium-...

Scheme 8: (A) Known biological activities related to cyclohexylamine derivatives; (B) an example of an intram...

Scheme 9: (A) Known biologically active benzophenone derivatives; (B and C) photocatalytic oxidation of benzy...

Scheme 10: (A) Known bioactive fluorine-containing compounds; (B and C) vanadium-mediated C(sp3)–H fluorinatio...

Scheme 11: (A) Known biologically active Lythraceae alkaloids; (B) synthesis of (±)-decinine (30).

Scheme 12: (A) Synthesis of (R)- and (S)-boehmeriasin (31); (B) synthesis of phenanthroindolizidines by vanadi...

Scheme 13: (A) Known bioactive BINOL derivatives; (B and C) vanadium-mediated oxidative coupling of 2-naphthol...

Scheme 14: (A) Known antiplasmodial imidazopyridazines; (B) practical synthesis of 41.

Scheme 15: (A) Gold-catalyzed drug-release mechanism using 2-alkynylbenzamides; (B and C) chromium-mediated al...

Scheme 16: (A) Examples of anti-inflammatory benzaldehyde derivatives; (B and C) chromium-mediated difunctiona...

Scheme 17: (A and B) Manganese-catalyzed chemoselective intramolecular C(sp3)–H amination; (C) late-stage modi...

Scheme 18: (A and B) Manganese-catalyzed C(sp3)–H amination; (C) late-stage modification of a leelamine deriva...

Scheme 19: (A) Known bioactive compounds containing substituted N-heterocycles; (B and C) manganese-catalyzed ...

Scheme 20: (A) Known indoles that present GPR40 full agonist activity; (B and C) manganese-catalyzed C–H alkyl...

Scheme 21: (A) Examples of known biaryl-containing drugs; (B and C) manganese-catalyzed C–H arylation through ...

Scheme 22: (A) Known zidovudine derivatives with potent anti-HIV properties; (B and C) manganese-catalyzed C–H...

Scheme 23: (A and B) Manganese-catalyzed C–H organic photo-electrosynthesis; (C) late-stage modification.

Scheme 24: (A) Example of a known antibacterial silylated dendrimer; (B and C) manganese-catalyzed C–H silylat...

Scheme 25: (A and B) Fe-based small molecule catalyst applied for selective aliphatic C–H oxidations; (C) late...

Scheme 26: (A) Examples of naturally occurring gracilioethers; (B) the first total synthesis of gracilioether ...

Scheme 27: (A and B) Selective aliphatic C–H oxidation of amino acids; (C) late-stage modification of proline-...

Scheme 28: (A) Examples of Illicium sesquiterpenes; (B) first chemical synthesis of (+)-pseudoanisatin (80) in...

Scheme 29: (A and B) Fe-catalyzed deuteration; (C) late-stage modification of pharmaceuticals.

Scheme 30: (A and B) Biomimetic Fe-catalyzed aerobic oxidation of methylarenes to benzaldehydes (PMHS, polymet...

Scheme 31: (A) Known tetrahydroquinolines with potential biological activities; (B and C) redox-selective Fe c...

Scheme 32: (A) Known drugs containing a benzofuran unit; (B and C) Fe/Cu-catalyzed tandem O-arylation to acces...

Scheme 33: (A) Known azaindolines that act as M4 muscarinic acetylcholine receptor agonists; (B and C) intramo...

Scheme 34: (A) Known indolinones with anticholinesterase activity; (B and C) oxidative C(sp3)–H cross coupling...

Scheme 35: (A and B) Cobalt-catalyzed C–H alkenylation of C-3-peptide-containing indoles; (C) derivatization b...

Scheme 36: (A) Cobalt-Cp*-catalyzed C–H methylation of known drugs; (B and C) scope of the o-methylated deriva...

Scheme 37: (A) Known lasalocid A analogues; (B and C) three-component cobalt-catalyzed C–H bond addition; (D) ...

Scheme 38: (A and B) Cobalt-catalyzed C(sp2)–H amidation of thiostrepton.

Scheme 39: (A) Known 4H-benzo[d][1,3]oxazin-4-one derivatives with hypolipidemic activity; (B and C) cobalt-ca...

Scheme 40: (A and B) Cobalt-catalyzed C–H arylation of pyrrole derivatives; (C) application for the synthesis ...

Scheme 41: (A) Known 2-phenoxypyridine derivatives with potent herbicidal activity; (B and C) cobalt-catalyzed...

Scheme 42: (A) Natural cinnamic acid derivatives; (B and C) cobalt-catalyzed C–H carboxylation of terminal alk...

Scheme 43: (A and B) Cobalt-catalyzed C–H borylation; (C) application to the synthesis of flurbiprofen.

Scheme 44: (A) Benzothiazoles known to present anticonvulsant activities; (B and C) cobalt/ruthenium-catalyzed...

Scheme 45: (A and B) Cobalt-catalyzed oxygenation of methylene groups towards ketone synthesis; (C) synthesis ...

Scheme 46: (A) Known anticancer tetralone derivatives; (B and C) cobalt-catalyzed C–H difluoroalkylation of ar...

Scheme 47: (A and B) Cobalt-catalyzed C–H thiolation; (C) application in the synthesis of quetiapine (153).

Scheme 48: (A) Known benzoxazole derivatives with anticancer, antifungal, and antibacterial activities; (B and...

Scheme 49: (A and B) Cobalt-catalyzed C–H carbonylation of naphthylamides; (C) BET inhibitors 158 and 159 tota...

Scheme 50: (A) Known bioactive pyrrolo[1,2-a]quinoxalin-4(5H)-one derivatives; (B and C) cobalt-catalyzed C–H ...

Scheme 51: (A) Known antibacterial cyclic sulfonamides; (B and C) cobalt-catalyzed C–H amination of propargyli...

Scheme 52: (A and B) Cobalt-catalyzed intramolecular 1,5-C(sp3)–H amination; (C) late-stage functionalization ...

Scheme 53: (A and B) Cobalt-catalyzed C–H/C–H cross-coupling between benzamides and oximes; (C) late-state syn...

Scheme 54: (A) Known anticancer natural isoquinoline derivatives; (B and C) cobalt-catalyzed C(sp2)–H annulati...

Scheme 55: (A) Enantioselective intramolecular nickel-catalyzed C–H activation; (B) bioactive obtained motifs;...

Scheme 56: (A and B) Nickel-catalyzed α-C(sp3)–H arylation of ketones; (C) application of the method using kno...

Scheme 57: (A and B) Nickel-catalyzed C(sp3)–H acylation of pyrrolidine derivatives; (C) exploring the use of ...

Scheme 58: (A) Nickel-catalyzed C(sp3)–H arylation of dioxolane; (B) library of products obtained from biologi...

Scheme 59: (A) Intramolecular enantioselective nickel-catalyzed C–H cycloalkylation; (B) product examples, inc...

Scheme 60: (A and B) Nickel-catalyzed C–H deoxy-arylation of azole derivatives; (C) late-stage functionalizati...

Scheme 61: (A and B) Nickel-catalyzed decarbonylative C–H arylation of azole derivatives; (C) application of t...

Scheme 62: (A and B) Another important example of nickel-catalyzed C–H arylation of azole derivatives; (C) app...

Scheme 63: (A and B) Another notable example of a nickel-catalyzed C–H arylation of azole derivatives; (C) lat...

Scheme 64: (A and B) Nickel-based metalorganic framework (MOF-74-Ni)-catalyzed C–H arylation of azole derivati...

Scheme 65: (A) Known commercially available benzothiophene-based drugs; (B and C) nickel-catalyzed C–H arylati...

Scheme 66: (A) Known natural tetrahydrofuran-containing substances; (B and C) nickel-catalyzed photoredox C(sp3...

Scheme 67: (A and B) Another notable example of a nickel-catalyzed photoredox C(sp3)–H alkylation/arylation; (...

Scheme 68: (A) Electrochemical/nickel-catalyzed C–H alkoxylation; (B) achieved scope, including three using na...

Scheme 69: (A) Enantioselective photoredox/nickel catalyzed C(sp3)–H arylation; (B) achieved scope, including ...

Scheme 70: (A) Known commercially available trifluoromethylated drugs; (B and C) nickel-catalyzed C–H trifluor...

Scheme 71: (A and B) Stereoselective nickel-catalyzed C–H difluoroalkylation; (C) late-stage functionalization...

Scheme 72: (A) Cu-mediated ortho-amination of oxalamides; (B) achieved scope, including derivatives obtained f...

Scheme 73: (A) Electro-oxidative copper-mediated amination of 8-aminoquinoline-derived amides; (B) achieved sc...

Scheme 74: (A and B) Cu(I)-mediated C–H amination with oximes; (C) derivatization using telmisartan (241) as s...

Scheme 75: (A and B) Cu-mediated amination of aryl amides using ammonia; (C) late-stage modification of proben...

Scheme 76: (A and B) Synthesis of purine nucleoside analogues using copper-mediated C(sp2)–H activation.

Scheme 77: (A) Copper-mediated annulation of acrylamide; (B) achieved scope, including the synthesis of the co...

Scheme 78: (A) Known bioactive compounds containing a naphthyl aryl ether motif; (B and C) copper-mediated eth...

Scheme 79: (A and B) Cu-mediated alkylation of N-oxide-heteroarenes; (C) late-stage modification.

Scheme 80: (A) Cu-mediated cross-dehydrogenative coupling of polyfluoroarenes and alkanes; (B) scope from know...

Scheme 81: (A) Known anticancer acrylonitrile compounds; (B and C) Copper-mediated cyanation of unactivated al...

Scheme 82: (A) Cu-mediated radiofluorination of 8-aminoquinoline-derived aryl amides; (B) achieved scope, incl...

Scheme 83: (A) Examples of natural β-carbolines; (B and C) an example of a zinc-catalyzed C–H functionalizatio...

Scheme 84: (A) Examples of anticancer α-aminophosphonic acid derivatives; (B and C) an example of a zinc-catal...
N-tert-Butanesulfinyl imines in the asymmetric synthesis of nitrogen-containing heterocycles
- Joseane A. Mendes,
- Paulo R. R. Costa,
- Miguel Yus,
- Francisco Foubelo and
- Camilla D. Buarque
Beilstein J. Org. Chem. 2021, 17, 1096–1140, doi:10.3762/bjoc.17.86

- imines derived from tert-butanesulfinamide. These imines are versatile chiral auxiliaries and have been extensively used as eletrophiles in a wide range of reactions. The electron-withdrawing sulfinyl group facilitates the nucleophilic addition of organometallic compounds to the iminic carbon with high
- ligands [52][53][54][55] and as chiral auxiliaries [56]. The most widely used synthetic methods to form the aziridine ring [57][58][59][60][61] include intramolecular cyclizations in amines bearing potential leaving groups. Stereoselective syntheses of aziridines have been successfully carried out by

Graphical Abstract

Scheme 1: General strategy for the enantioselective synthesis of N-containing heterocycles from N-tert-butane...

Scheme 2: Methodologies for condensation of aldehydes and ketones with tert-butanesulfinamides (1).

Scheme 3: Transition models for cis-aziridines and trans-aziridines.

Scheme 4: Mechanism for the reduction of N-tert-butanesulfinyl imines.

Scheme 5: Transition models for the addition of organomagnesium and organolithium compounds to N-tert-butanes...

Scheme 6: Synthesis of 2,2-dibromoaziridines 15 from aldimines 14 and bromoform, and proposed non-chelation-c...

Scheme 7: Diastereoselective synthesis of aziridines from tert-butanesulfinyl imines.

Scheme 8: Synthesis of vinylaziridines 22 from aldimines 14 and 1,3-dibromopropene 23, and proposed chelation...

Scheme 9: Synthesis of vinylaziridines 27 from aldimines 14 and α-bromoesters 26, and proposed transition sta...

Scheme 10: Synthesis of 2-chloroaziridines 28 from aldimines 14 and dichloromethane, and proposed transition s...

Scheme 11: Synthesis of cis-vinylaziridines 30 and 31 from aldimines 14 and bromomethylbutenolide 29.

Scheme 12: Synthesis of 2-chloro-2-aroylaziridines 36 and 32 from aldimines 14, arylnitriles 34, and silyldich...

Scheme 13: Synthesis of trifluoromethylaziridines 39 and proposed transition state of the aziridination.

Scheme 14: Synthesis of aziridines 42 and proposed state transition.

Scheme 15: Synthesis of 1-substituted 2-azaspiro[3.3]heptanes, 1-phenyl-2-azaspiro[3.4]octane and 1-phenyl-2-a...

Scheme 16: Synthesis of 1-substituted 2,6-diazaspiro[3.3]heptanes 48 from chiral imines 14 and 1-Boc-azetidine...

Scheme 17: Synthesis of β-lactams 52 from chiral imines 14 and dimethyl malonate (49).

Scheme 18: Synthesis of spiro-β-lactam 57 from chiral (RS)-N-tert-butanesulfinyl isatin ketimine 53 and ethyl ...

Scheme 19: Synthesis of β-lactam 60, a precursor of (−)-batzelladine D (61) and (−)-13-epi-batzelladine D (62)...

Scheme 20: Rhodium-catalyzed asymmetric synthesis of 3-substituted pyrrolidines 66 from chiral imine (RS)-63 a...

Scheme 21: Asymmetric synthesis of 1,3-disubstituted isoindolines 69 and 70 from chiral imine 67.

Scheme 22: Asymmetric synthesis of cis-2,5-disubstituted pyrrolidines 73 from chiral imine (RS)-71.

Scheme 23: Asymmetric synthesis of 3-hydroxy-5-substituted pyrrolidin-2-ones 77 from chiral imine (RS)-74.

Scheme 24: Asymmetric synthesis of 4-hydroxy-5-substituted pyrrolidin-2-ones 80 from chiral imines 79.

Scheme 25: Asymmetric synthesis of 3-pyrrolines 82 from chiral imines 14 and ethyl 4-bromocrotonate (81).

Scheme 26: Asymmetric synthesis of γ-amino esters 84, and tetramic acid derivative 86 from chiral imines (RS)-...

Scheme 27: Asymmetric synthesis of α-methylene-γ-butyrolactams 90 from chiral imines (Z,SS)-87 and ethyl 2-bro...

Scheme 28: Asymmetric synthesis of methylenepyrrolidines 92 from chiral imines (RS)-14 and 2-(trimethysilylmet...

Scheme 29: Synthesis of dibenzoazaspirodecanes from cyclic N-tert-butanesulfinyl imines.

Scheme 30: Stereoselective synthesis of cyclopenta[c]proline derivatives 103 from β,γ-unsaturated α-amino acid...

Scheme 31: Stereoselective synthesis of alkaloids (−)-angustureine (107) and (−)-cuspareine (108).

Scheme 32: Stereoselective synthesis of alkaloids (−)-pelletierine (112) and (+)-coniine (117).

Scheme 33: Synthesis of piperidine alkaloids (+)-dihydropinidine (122a), (+)-isosolenopsin (122b) and (+)-isos...

Scheme 34: Stereoselective synthesis of the alkaloids(+)-sedamine (125) from chiral imine (SS)-119.

Scheme 35: Stereoselective synthesis of trans-5-hydroxy-6-substituted-2-piperidinones 127 and 129 from chiral ...

Scheme 36: Stereoselective synthesis of trans-5-hydroxy-6-substituted ethanone-2-piperidinones 132 from chiral...

Scheme 37: Stereoselective synthesis of trans-3-benzyl-5-hydroxy-6-substituted-2-piperidinones 136 from chiral...

Scheme 38: Stereoselective synthesis of trans-5-hydroxy-6-substituted 2-piperidinones 139 from chiral imine 138...

Scheme 39: Stereoselective synthesis of ʟ-hydroxypipecolic acid 145 from chiral imine 144.

Scheme 40: Synthesis of 1-substituted isoquinolones 147, 149 and 151.

Scheme 41: Stereoselective synthesis of 3-substituted dihydrobenzo[de]isoquinolinones 154.

Scheme 42: Enantioselective synthesis of alkaloids (S)-1-benzyl-6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline (...

Scheme 43: Enantioselective synthesis of alkaloids (−)-cermizine B (171) and (+)-serratezomine E (172) develop...

Scheme 44: Stereoselective synthesis of (+)-isosolepnosin (177) and (+)-solepnosin (178) from homoallylamine d...

Scheme 45: Stereoselective synthesis of tetrahydroquinoline derivatives 184, 185 and 187 from chiral imines (RS...

Scheme 46: Stereoselective synthesis of pyridobenzofuran and pyridoindole derivatives 193 from homopropargylam...

Scheme 47: Stereoselective synthesis of 2-substituted 1,2,5,6-tetrahydropyridines 196 from chiral imines (RS)-...

Scheme 48: Stereoselective synthesis of 2-substituted trans-2,6-disubstituted piperidine 199 from chiral imine...

Scheme 49: Stereoselective synthesis of cis-2,6-disubstituted piperidines 200, and alkaloid (+)-241D, from chi...

Scheme 50: Stereoselective synthesis of 6-substituted piperidines-2,5-diones 206 and 1,7-diazaspiro[4.5]decane...

Scheme 51: Stereoselective synthesis of spirocyclic oxindoles 210 from chiral imines (RS)-53.

Scheme 52: Stereoselective synthesis of azaspiro compound 213 from chiral imine 211.

Scheme 53: Stereoselective synthesis of tetrahydroisoquinoline derivatives from chiral imines (RS)-214.

Scheme 54: Stereoselective synthesis of (−)-crispine A 223 from chiral imine (RS)-214.

Scheme 55: Synthesis of (−)-harmicine (228) using tert-butanesulfinamide through haloamide cyclization.

Scheme 56: Stereoselective synthesis of tetraponerines T1–T8.

Scheme 57: Stereoselective synthesis of phenanthroindolizidines 246a and (−)-tylophorine (246b), and phenanthr...

Scheme 58: Stereoselective synthesis of indoline, tetrahydroquinoline and tetrahydrobenzazepine derivatives 253...

Scheme 59: Stereoselective synthesis of (+)-epohelmin A (258) and (+)-epohelmin B (260) from aldimine (RS)-79.

Scheme 60: Stereoselective synthesis of (−)-epiquinamide (266) from chiral aldimine (SS)-261.

Scheme 61: Synthesis synthesis of (–)-hippodamine (273) and (+)-epi-hippodamine (272) using chiral sulfinyl am...

Scheme 62: Stereoselective synthesis of (+)-grandisine D (279) and (+)-amabiline (283).

Scheme 63: Stereoselective synthesis of (−)-epiquinamide (266) and (+)-swaisonine (291) from aldimine (SS)-126....

Scheme 64: Stereoselective synthesis of (+)-C(9a)-epi-epiquinamide (294).

Scheme 65: Stereoselective synthesis of (+)-lasubine II (298) from chiral aldimine (SS)-109.

Scheme 66: Stereoselective synthesis of (−)-epimyrtine (300a) and (−)-lasubine II (ent-302) from β-amino keton...

Scheme 67: Stereoselective synthesis of (−)-tabersonine (310), (−)-vincadifformine (311), and (−)-aspidospermi...

Scheme 68: Stereoselective synthesis of (+)-epohelmin A (258) and (+)-epohelmin B (260) from aldehyde 313 and ...

Scheme 69: Total synthesis of (+)-lysergic acid (323) from N-tert-butanesulfinamide (RS)-1.
CF3-substituted carbocations: underexploited intermediates with great potential in modern synthetic chemistry
- Anthony J. Fernandes,
- Armen Panossian,
- Bastien Michelet,
- Agnès Martin-Mingot,
- Frédéric R. Leroux and
- Sébastien Thibaudeau
Beilstein J. Org. Chem. 2021, 17, 343–378, doi:10.3762/bjoc.17.32

- a trifluoromethylated iminium ion 187 during the course of their studies on a Strecker-type reaction [123]. Starting from trifluoromethylated imines 193 or oxazolidines 194 and 195 bearing enantiopure chiral auxiliaries, the authors accessed the corresponding cyano derivatives 196–198 with different

Graphical Abstract

Figure 1: Stabilizing interaction in the CF3CH2+ carbenium ion (top) and structure of the first observable fl...

Scheme 1: Isodesmic equations accounting for the destabilizing effect of the CF3 group. ΔE in kcal⋅mol−1, cal...

Scheme 2: Stabilizing effect of fluorine atoms by resonance electron donation in carbenium ions (δ in ppm).

Scheme 3: Direct in situ NMR observation of α-(trifluoromethyl)carbenium ion or protonated alcohols. Δδ = δ19...

Scheme 4: Reported 13C NMR chemical shifts for the α-(trifluoromethyl)carbenium ion 10c (δ in ppm).

Scheme 5: Direct NMR observation of α-(trifluoromethyl)carbenium ions in situ (δ in ppm).

Scheme 6: Illustration of the ion pair solvolysis mechanism for sulfonate 13f. YOH = solvent.

Figure 2: Solvolysis rate for 13a–i and 17.

Figure 3: Structures of allyl triflates 18 and 19 and allyl brosylate 20. Bs = p-BrC6H4SO2.

Figure 4: Structure of tosylate derivatives 21.

Figure 5: a) Structure of triflate derivatives 22. b) Stereochemistry outcomes of the reaction starting from (...

Scheme 7: Solvolysis reaction of naphthalene and anthracenyl derivatives 26 and 29.

Figure 6: Structure of bisarylated derivatives 34.

Figure 7: Structure of bisarylated derivatives 36.

Scheme 8: Reactivity of 9c in the presence of a Brønsted acid.

Scheme 9: Cationic electrocyclization of 38a–c under strongly acidic conditions.

Scheme 10: Brønsted acid-catalyzed synthesis of indenes 42 and indanes 43.

Scheme 11: Reactivity of sulfurane 44 in triflic acid.

Scheme 12: Solvolysis of triflate 45f in alcoholic solvents.

Scheme 13: Synthesis of labeled 18O-52.

Scheme 14: Reactivity of sulfurane 53 in triflic acid.

Figure 8: Structure of tosylates 56 and 21f.

Scheme 15: Resonance forms in benzylic carbenium ions.

Figure 9: Structure of pyrrole derivatives 58 and 59.

Scheme 16: Resonance structure 60↔60’.

Scheme 17: Ga(OTf)3-catalyzed synthesis of 3,3’- and 3,6’-bis(indolyl)methane from trifluoromethylated 3-indol...

Scheme 18: Proposed reaction mechanism.

Scheme 19: Metal-free 1,2-phosphorylation of 3-indolylmethanols.

Scheme 20: Superacid-mediated arylation of thiophene derivatives.

Scheme 21: In situ mechanistic NMR investigations.

Scheme 22: Proposed mechanisms for the prenyltransferase-catalyzed condensation.

Scheme 23: Influence of a CF3 group on the allylic SN1- and SN2-mechanism-based reactions.

Scheme 24: Influence of the CF3 group on the condensation reaction.

Scheme 25: Solvolysis of 90 in TFE.

Scheme 26: Solvolysis of allyl triflates 94 and 97 and isomerization attempt of 96.

Scheme 27: Proposed mechanism for the formation of 95.

Scheme 28: Formation of α-(trifluoromethyl)allylcarbenium ion 100 in a superacid.

Scheme 29: Lewis acid activation of CF3-substituted allylic alcohols.

Scheme 30: Bimetallic-cluster-stabilized α-(trifluoromethyl)carbenium ions.

Scheme 31: Reactivity of cluster-stabilized α-(trifluoromethyl)carbenium ions.

Scheme 32: α-(Trifluoromethyl)propargylium ion 122↔122’ generated from silyl ether 120 in a superacid.

Scheme 33: Formation of α-(trifluoromethyl)propargylium ions from CF3-substituted propargyl alcohols.

Scheme 34: Direct NMR observation of the protonation of some trifluoromethyl ketones in situ and the correspon...

Scheme 35: Selected resonance forms in protonated fluoroketone derivatives.

Scheme 36: Acid-catalyzed Friedel–Crafts reactions of trifluoromethyl ketones 143a,b and 147a–c.

Scheme 37: Enantioselective hydroarylation of CF3-substituted ketones.

Scheme 38: Acid-catalyzed arylation of ketones 152a–c.

Scheme 39: Reactivity of 156 in a superacid.

Scheme 40: Reactivity of α-CF3-substituted heteroaromatic ketones and alcohols as well as 1,3-diketones.

Scheme 41: Reactivity of 168 with benzene in the presence of a Lewis or Brønsted acid.

Scheme 42: Acid-catalyzed three-component asymmetric reaction.

Scheme 43: Anodic oxidation of amines 178a–c and proposed mechanism.

Scheme 44: Reactivity of 179b in the presence of a strong Lewis acid.

Scheme 45: Trifluoromethylated derivatives as precursors of trifluoromethylated iminium ions.

Scheme 46: Mannich reaction with trifluoromethylated hemiaminal 189.

Scheme 47: Suitable nucleophiles reacting with 192 after Lewis acid activation.

Scheme 48: Strecker reaction involving the trifluoromethylated iminium ion 187.

Scheme 49: Reactivity of 199 toward nucleophiles.

Scheme 50: Reactivity of 204a with benzene in the presence of a Lewis acid.

Scheme 51: Reactivity of α-(trifluoromethyl)-α-chloro sulfides in the presence of strong Lewis acids.

Scheme 52: Anodic oxidation of sulfides 213a–h and Pummerer rearrangement.

Scheme 53: Mechanism for the electrochemical oxidation of the sulfide 213a.

Scheme 54: Reactivity of (trifluoromethyl)diazomethane (217a) in HSO3F.

Figure 10: a) Structure of diazoalkanes 217a–c and b) rate-limiting steps of their decomposition.

Scheme 55: Deamination reaction of racemic 221 and enantioenriched (S)-221.

Scheme 56: Deamination reaction of labeled 221-d2. Elimination products were formed in this reaction, the yiel...

Scheme 57: Deamination reaction of 225-d2. Elimination products were also formed in this reaction in undetermi...

Scheme 58: Formation of 229 from 228 via 1,2-H-shift.

Scheme 59: Deamination reaction of 230. Elimination products were formed in this reaction, the yield of which ...

Scheme 60: Deamination of several diazonium ions. Elimination products were formed in these reactions, the yie...

Scheme 61: Solvolysis reaction mechanism of alkyl tosylates.

Scheme 62: Solvolysis outcome for the tosylates 248 and 249 in HSO3FSbF5.

Figure 11: Solvolysis rate of 248, 249, 252, and 253 in 91% H2SO4.

Scheme 63: Illustration of the reaction pathways. TsCl, pyridine, −5 °C (A); 98% H2SO4, 30 °C (B); 98% H2SO4, ...

Scheme 64: Proposed solvolysis mechanism for the aliphatic tosylate 248.

Scheme 65: Solvolysis of the derivatives 259 and 260.

Scheme 66: Solvolysis of triflate 261. SOH = solvent.

Scheme 67: Intramolecular Friedel–Crafts alkylations upon the solvolysis of triflates 264 and 267.

Scheme 68: α-CF3-enhanced γ-silyl elimination of cyclobutyltosylates 270a,b.

Scheme 69: γ-Silyl elimination in the synthesis of a large variety of CF3-substituted cyclopropanes. Pf = pent...

Scheme 70: Synthetic pathways to 281. aNMR yields.

Scheme 71: The cyclopropyl-substituted homoallylcyclobutylcarbenium ion manifold.

Scheme 72: Reactivity of CF3-substituted cyclopropylcarbinyl derivatives 287a–c. LG = leaving group.

Scheme 73: Reactivity of CF3-substituted cyclopropylcarbinyl derivatives 291a–c.

Scheme 74: Superacid-promoted dimerization or TFP.

Scheme 75: Reactivity of TFP in a superacid.

Scheme 76: gem-Difluorination of α-fluoroalkyl styrenes via the formation of a “hidden” α-RF-substituted carbe...

Scheme 77: Solvolysis of CF3-substituted pentyne 307.

Scheme 78: Photochemical rearrangement of 313.

Figure 12: Structure of 2-norbornylcarbenium ion 318 and argued model for the stabilization of this cation.

Figure 13: Structures and solvolysis rate (TFE, 25 °C) of the sulfonates 319–321. Mos = p-MeOC6H4SO2.

Scheme 79: Mechanism for the solvolysis of 323. SOH = solvent.

Scheme 80: Products formed by the hydrolysis of 328.

Scheme 81: Proposed carbenium ion intermediates in an equilibrium during the solvolysis of tosylates 328, 333,...
The preparation and properties of 1,1-difluorocyclopropane derivatives
- Kymbat S. Adekenova,
- Peter B. Wyatt and
- Sergazy M. Adekenov
Beilstein J. Org. Chem. 2021, 17, 245–272, doi:10.3762/bjoc.17.25
- cyclopropane formation did not require Et3B (Scheme 25). The work was extended to include boron-free, diastereoselective versions incorporating N-acylimidazolidinone chiral auxiliaries (Scheme 26). Cyclization reaction of phenylacetonitrile and 1,2-dibromo-1,1-difluoroethane: Kagabu et al. showed that the

Graphical Abstract

Scheme 1: Synthesis of 1,1-difluoro-2,3-dimethylcyclopropane (2).

Scheme 2: Cyclopropanation via dehydrohalogenation of chlorodifluoromethane.

Scheme 3: Difluorocyclopropanation of methylstyrene 7 using dibromodifluoromethane and zinc.

Scheme 4: Synthesis of difluorocyclopropanes from the reaction of dibromodifluoromethane and triphenylphosphi...

Scheme 5: Generation of difluorocarbene in a catalytic two-phase system and its addition to tetramethylethyle...

Scheme 6: The reaction of methylstyrene 7 with chlorodifluoromethane (11) in the presence of a tetraarylarson...

Scheme 7: Pyrolysis of sodium chlorodifluoroacetate (12) in refluxing diglyme in the presence of alkene 13.

Scheme 8: Synthesis of boron-substituted gem-difluorocyclopropanes 16.

Scheme 9: Addition of sodium bromodifluoroacetate (17) to alkenes.

Scheme 10: Addition of sodium bromodifluoroacetate (17) to silyloxy-substituted cyclopropanes 20.

Scheme 11: Synthesis of difluorinated nucleosides.

Scheme 12: Addition of butyl acrylate (26) to difluorocarbene generated from TFDA (25).

Scheme 13: Addition of difluorocarbene to propargyl esters 27 and conversion of the difluorocyclopropenes 28 t...

Scheme 14: The generation of difluorocyclopropanes using MDFA 30.

Scheme 15: gem-Difluorocyclopropanation of styrene (32) using difluorocarbene generated from TMSCF3 (31) under...

Scheme 16: Synthesis of a gem-difluorocyclopropane derivative using HFPO (41) as a source of difluorocarbene.

Scheme 17: Cyclopropanation of (Z)-2-butene in the presence of difluorodiazirine (44).

Scheme 18: The cyclopropanation of 1-octene (46) using Seyferth's reagent (45) as a source of difluorocarbene.

Scheme 19: Alternative approaches for the difluorocarbene synthesis from trimethyl(trifluoromethyl)tin (48).

Scheme 20: Difluorocyclopropanation of cyclohexene (49).

Scheme 21: Synthesis of difluorocyclopropane derivative 53 using bis(trifluoromethyl)cadmium (51) as the diflu...

Scheme 22: Addition of difluorocarbene generated from tris(trifluoromethyl)bismuth (54).

Scheme 23: Addition of a stable (trifluoromethyl)zinc reagent to styrenes.

Scheme 24: The preparation of 2,2-difluorocyclopropanecarboxylic acids of type 58.

Scheme 25: Difluorocyclopropanation via Michael cyclization.

Scheme 26: Difluorocyclopropanation using N-acylimidazolidinone 60.

Scheme 27: Difluorocyclopropanation through the cyclization of phenylacetonitrile (61) and 1,2-dibromo-1,1-dif...

Scheme 28: gem-Difluoroolefins 64 for the synthesis of functionalized cyclopropanes 65.

Scheme 29: Preparation of aminocyclopropanes 70.

Scheme 30: Synthesis of fluorinated methylenecyclopropane 74 via selenoxide elimination.

Scheme 31: Reductive dehalogenation of (1R,3R)-75.

Scheme 32: Synthesis of chiral monoacetates by lipase catalysis.

Scheme 33: Transformation of (±)-trans-81 using Rhodococcus sp. AJ270.

Scheme 34: Transformation of (±)-trans-83 using Rhodococcus sp. AJ270.

Scheme 35: Hydrogenation of difluorocyclopropenes through enantioselective hydrocupration.

Scheme 36: Enantioselective transfer hydrogenation of difluorocyclopropenes with a Ru-based catalyst.

Scheme 37: The thermal transformation of trans-1,2-dichloro-3,3-difluorocyclopropane (84).

Scheme 38: cis–trans-Epimerization of 1,1-difluoro-2,3-dimethylcyclopropane.

Scheme 39: 2,2-Difluorotrimethylene diradical intermediate.

Scheme 40: Ring opening of stereoisomers 88 and 89.

Scheme 41: [1,3]-Rearrangement of alkenylcyclopropanes 90–92.

Scheme 42: Thermolytic rearrangement of 2,2-difluoro-1-vinylcyclopropane (90).

Scheme 43: Thermal rearrangement for ethyl 3-(2,2-difluoro)-3-phenylcyclopropyl)acrylates 93 and 95.

Scheme 44: Possible pathways of the ring opening of 1,1-difluoro-2-vinylcyclopropane.

Scheme 45: Equilibrium between 1,1-difluoro-2-methylenecyclopropane (96) and (difluoromethylene)cyclopropane 97...

Scheme 46: Ring opening of substituted 1,1-difluoro-2,2-dimethyl-3-methylenecyclopropane 98.

Scheme 47: 1,1-Difluorospiropentane rearrangement.

Scheme 48: Acetolysis of (2,2-difluorocyclopropyl)methyl tosylate (104) and (1,1-difluoro-2-methylcyclopropyl)...

Scheme 49: Ring opening of gem-difluorocyclopropyl ketones 106 and 108 by thiolate nucleophiles.

Scheme 50: Hydrolysis of gem-difluorocyclopropyl acetals 110.

Scheme 51: Ring-opening reaction of 2,2-difluorocyclopropyl ketones 113 in the presence of ionic liquid as a s...

Scheme 52: Ring opening of gem-difluorocyclopropyl ketones 113a by MgI2-initiated reaction with diarylimines 1...

Scheme 53: Ring-opening reaction of gem-difluorocyclopropylstannanes 117.

Scheme 54: Preparation of 1-fluorovinyl vinyl ketone 123 and the synthesis of 2-fluorocyclopentenone 124. TBAT...

Scheme 55: Iodine atom-transfer ring opening of 1,1-difluoro-2-(1-iodoalkyl)cyclopropanes 125a–c.

Scheme 56: Ring opening of bromomethyl gem-difluorocyclopropanes 130 and formation of gem-difluoromethylene-co...

Scheme 57: Ring-opening aerobic oxidation reaction of gem-difluorocyclopropanes 132.

Scheme 58: Dibrominative ring-opening functionalization of gem-difluorocyclopropanes 134.

Scheme 59: The selective formation of (E,E)- and (E,Z)-fluorodienals 136 and 137 from difluorocyclopropyl acet...

Scheme 60: Proposed mechanism for the reaction of difluoro(methylene)cyclopropane 139 with Br2.

Scheme 61: Thermal rearrangement of F2MCP 139 and iodine by CuI catalysis.

Scheme 62: Synthesis of 2-fluoropyrroles 142.

Scheme 63: Ring opening of gem-difluorocyclopropyl ketones 143 mediated by BX3.

Scheme 64: Lewis acid-promoted ring-opening reaction of 2,2-difluorocyclopropanecarbonyl chloride (148).

Scheme 65: Ring-opening reaction of the gem-difluorocyclopropyl ketone 106 by methanolic KOH.

Scheme 66: Hydrogenolysis of 1,1-difluoro-3-methyl-2-phenylcyclopropane (151).

Scheme 67: Synthesis of monofluoroalkenes 157.

Scheme 68: The stereoselective Ag-catalyzed defluorinative ring-opening diarylation of 1-trimethylsiloxy-2,2-d...

Scheme 69: Synthesis of 2-fluorinated allylic compounds 162.

Scheme 70: Pd-catalyzed cross-coupling reactions of gem-difluorinated cyclopropanes 161.

Scheme 71: The (Z)-selective Pd-catalyzed ring-opening sulfonylation of 2-(2,2-difluorocyclopropyl)naphthalene...

Figure 1: Structures of zosuquidar hydrochloride and PF-06700841.

Scheme 72: Synthesis of methylene-gem-difluorocyclopropane analogs of nucleosides.

Figure 2: Anthracene-difluorocyclopropane hybrid derivatives.

Figure 3: Further examples of difluorcyclopropanes in modern drug discovery.
The biomimetic synthesis of balsaminone A and ellagic acid via oxidative dimerization
- Sharna-kay Daley and
- Nadale Downer-Riley
Beilstein J. Org. Chem. 2020, 16, 2026–2031, doi:10.3762/bjoc.16.169
- ; ellagic acid; oxidative dimerization; Introduction Over the last century, the formation of an aryl to aryl bond has garnered considerable synthetic attention due to the applications of biaryls as pharmaceutical agents, as well as chiral auxiliaries in asymmetric synthesis [1][2][3]. Methods such as the

Graphical Abstract

Figure 1: Selected natural products synthesized via oxidative dimerization.

Scheme 1: Proposed biosynthesis of balsaminone A (4) [19].

Scheme 2: Proposed biosynthesis of ellagic acid (5) [20].

Scheme 3: Previous syntheses of balsaminone A (4) [22] and ellagic acid (5) [23].

Scheme 4: Attempted synthesis of the biomimetic precursor 9. [O]: Act-C, K3[Fe(CN)6], or p-benzoquinone.

Scheme 5: Biomimetic synthesis of balsaminone A (4).

Scheme 6: Concise and efficient biomimetic synthesis of ellagic acid (5).
One-pot synthesis of oxazolidinones and five-membered cyclic carbonates from epoxides and chlorosulfonyl isocyanate: theoretical evidence for an asynchronous concerted pathway
- Esra Demir,
- Ozlem Sari,
- Yasin Çetinkaya,
- Ufuk Atmaca,
- Safiye Sağ Erdem and
- Murat Çelik
Beilstein J. Org. Chem. 2020, 16, 1805–1819, doi:10.3762/bjoc.16.148
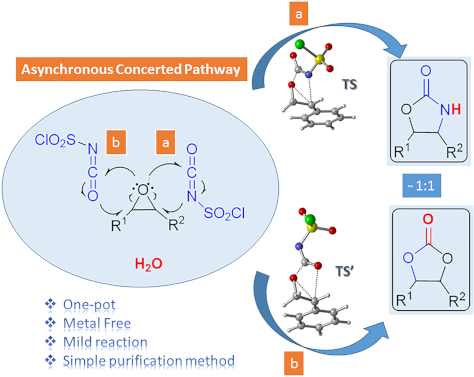
- considerable use as antibiotics [1], immunomodulators [2], antibacterials [3], as well as synthetic intermediates and chiral auxiliaries for various organic conversions [4][5][6][7]. Linezolid [1][2][3] (3) and cytoxazone [8][9] (4) are oxazolidinone derivatives having significant biological activities

Graphical Abstract

Scheme 1: Oxazolidinone (1), five-membered cyclic carbonate (2) and some important compounds containing an ox...

Scheme 2: Proposed mechanisms by Keshava Murthy and Dhar [41] and De Meijere and co-workers [42].

Figure 1: Possible pathways for the formation of oxazolidinone intermediates 10 and 11. Optimized transition ...

Figure 2: Potential energy profile related to the formation of oxazolidinone intermediates 10 and 11 at the P...

Figure 3: IRC calculated for the formation of (a) 10 and (b) 11 at M06-2X/6-31+G(d,p) level. I-1, I-15, I-35, ...

Figure 4: Optimized geometries for the stationary points for the formation of 10 at PCM(DCM)/M06-2X/6-31+G(d,...

Scheme 3: Proposed mechanisms for the formation of oxazolidinone 9f.

Figure 5: Potential energy profiles for paths 1a (blue), 1b (red), 2 (green) and relative Gibbs free energies...

Figure 6: Optimized geometries for the stationary points of path 1b at PCM(DCM)/M06-2X/6-31+G(d,p)//M06-2X/6-...

Scheme 4: Proposed mechanism for the formation of five-membered cyclic carbonate 8f.

Figure 7: Potential energy profile and relative Gibbs free energies (kcal/mol) in DCM related to the formatio...

Figure 8: Optimized geometries for the stationary points of step 1 for the formation of 16 at PCM(DCM)/M06-2X...

Figure 9: Optimized geometries for the stationary points of step 2 for the formation of 17 at PCM(DCM)/M06-2X...

Figure 10: Optimized geometries for the stationary points of step 3 for the formation of PC8 at PCM(DCM)/M06-2...
A review of asymmetric synthetic organic electrochemistry and electrocatalysis: concepts, applications, recent developments and future directions
- Munmun Ghosh,
- Valmik S. Shinde and
- Magnus Rueping
Beilstein J. Org. Chem. 2019, 15, 2710–2746, doi:10.3762/bjoc.15.264

- this area have established a number of asymmetric electrochemical inductors. After a thorough comprehension of the available literature, we propose to classify the chiral electrochemical inductors into three broad categories: chiral electrodes, chiral media and chiral auxiliaries [17]. We have
- 1994, Zielinski and Schäfer made a vital contribution in the field of asymmetric electrosynthesis in terms of the diastereoselective cathodic reduction of the carbonyl group of chiral phenylglyoxamides 150 and 152 [89][90]. After initial conversion to its corresponding amides using chiral auxiliaries
- phenylglyoxalic acid was further converted to mandelic acid derivatives 151 and 153, respectively, upon reduction at a mercury pool cathode. The electrolysis afforded an excellent chemical yield along with moderate to good diastereomeric excesses. Moreover, the recovery of chiral auxiliaries upon hydrolysis of

Graphical Abstract

Figure 1: General classification of asymmetric electroorganic reactions.

Scheme 1: Asymmetric reduction of 4-acetylpyridine using a modified graphite cathode.

Scheme 2: Asymmetric hydrogenation of ketones using Raney nickel powder electrodes modified with optically ac...

Scheme 3: Asymmetric reduction of prochiral activated olefins with a poly-ʟ-valine-coated graphite cathode.

Scheme 4: Asymmetric reduction of prochiral carbonyl compounds, oximes and gem-dibromides on a poly-ʟ-valine-...

Scheme 5: Asymmetric hydrogenation of prochiral ketones with poly[RuIII(L)2Cl2]+-modified carbon felt cathode...

Scheme 6: Asymmetric hydrogenation of α-keto esters using chiral polypyrrole film-coated cathode incorporated...

Scheme 7: Quinidine and cinchonidine alkaloid-induced asymmetric electroreduction of acetophenone.

Scheme 8: Asymmetric electroreduction of 4- and 2-acetylpyridines at a mercury cathode in the presence of a c...

Scheme 9: Enantioselective reduction of 4-methylcoumarin in the presence of catalytic yohimbine.

Scheme 10: Cinchonine-induced asymmetric electrocarboxylation of 4-methylpropiophenone.

Scheme 11: Enantioselective hydrogenation of methyl benzoylformate using an alkaloid entrapped silver cathode.

Scheme 12: Alkaloid-induced enantioselective hydrogenation using a Cu nanoparticle cathode.

Scheme 13: Alkaloid-induced enantioselective hydrogenation of aromatic ketones using a bimetallic Pt@Cu cathod...

Scheme 14: Enantioselective reduction of ketones at mercury cathode using N,N'-dimethylquininium tetrafluorobo...

Scheme 15: Asymmetric synthesis of an amino acid using an electrode modified with amino acid oxidase and elect...

Scheme 16: Asymmetric oxidation of p-tolyl methyl sulfide using chemically modified graphite anode.

Scheme 17: Asymmetric oxidation of unsymmetric sulfides using poly(amino acid)-coated electrodes.

Scheme 18: Enantioselective, electocatalytic oxidative coupling on TEMPO-modified graphite felt electrode in t...

Scheme 19: Asymmetric electrocatalytic oxidation of racemic alcohols on a TEMPO-modified graphite felt electro...

Scheme 20: Asymmetric electrocatalytic lactonization of diols on TEMPO-modified graphite felt electrodes.

Scheme 21: Asymmetric electrochemical pinacolization in a chiral solvent.

Scheme 22: Asymmetric electroreduction using a chiral supporting electrolyte.

Scheme 23: Asymmetric anodic oxidation of enol acetates using chiral supporting electrolytes.

Scheme 24: Kinetic resolution of primary amines using a chiral N-oxyl radical mediator.

Scheme 25: Chiral N-oxyl-radical-mediated kinetic resolution of secondary alcohols via electrochemical oxidati...

Scheme 26: Chiral iodoarene-mediated asymmetric electrochemical lactonization.

Scheme 27: Os-catalyzed electrochemical asymmetric dihydroxylation of olefins using the Sharpless ligand and i...

Scheme 28: Asymmetric electrochemical epoxidation of olefins catalyzed by a chiral Mn-salen complex.

Scheme 29: Asymmetric electrooxidation of 1,2-diols, and amino alcohols using a chiral copper catalyst.

Scheme 30: Mechanism of asymmetric electrooxidation of 1,2-diols, and amino alcohols using a chiral copper cat...

Scheme 31: Enantioselective electrocarboxylation catalyzed by an electrogenerated chiral [CoI(salen)]− complex....

Scheme 32: Asymmetric oxidative cross coupling of 2-acylimidazoles with silyl enol ethers.

Scheme 33: Ni-catalyzed asymmetric electroreductive cleavage of allylic β-keto ester 89.

Scheme 34: Asymmetric alkylation using a combination of electrosynthesis and a chiral Ni catalyst.

Scheme 35: Mechanism of asymmetric alkylation using a combination of electrosynthesis and a chiral Ni catalyst....

Scheme 36: Asymmetric epoxidation by electrogenerated percarbonate and persulfate ions in the presence of chir...

Scheme 37: α-Oxyamination of aldehydes via anodic oxidation catalyzed by chiral secondary amines.

Scheme 38: The α-alkylation of aldehydes via anodic oxidation catalyzed by chiral secondary amines.

Scheme 39: Mechanism of α-alkylation of aldehydes via anodic oxidation catalyzed by chiral secondary amines.

Scheme 40: Electrochemical chiral secondary amine-catalyzed intermolecular α-arylation of aldehydes.

Scheme 41: Mechanism of electrochemical chiral secondary amine-catalyzed intermolecular α-arylation of aldehyd...

Scheme 42: Asymmetric cross-dehydrogenative coupling of tertiary amines with simple ketones via an electrochem...

Scheme 43: Electroenzymatic asymmetric reduction using enoate reductase.

Scheme 44: Assymetric reduction using alcohol dehydrogenase as the electrocatalyst.

Scheme 45: Asymmetric electroreduction catalyzed by thermophilic NAD-dependent alcohol dehydrogenase.

Scheme 46: Asymmetric epoxidation of styrene by electrochemical regeneration of flavin-dependent monooxygenase....

Scheme 47: Asymmetric electroreduction using a chloroperoxidase catalyst.

Scheme 48: Asymmetric electrochemical transformation mediated by hydrophobic vitamin B12.

Scheme 49: Diastereoselective cathodic reduction of phenylglyoxalic acids substituted with amines as chiral au...

Scheme 50: Ni-catalyzed asymmetric electroreductive cross coupling of aryl halides with α-chloropropanoic acid...

Scheme 51: Electrochemical Mannich addition of silyloxyfuran to in situ-generated N-acyliminium ions.

Scheme 52: Stereoselective electroreductive homodimerization of cinnamates attached to a camphor-derived chira...

Scheme 53: Diastereoselective electrochemical carboxylation of chiral α-bromocarboxylic acid derivatives.

Scheme 54: Electrocatalytic stereoselective conjugate addition of chiral β-dicarbonyl compounds to methyl viny...

Scheme 55: Stereoselective electrochemical carboxylation of chiral cinnamic acid derivatives under a CO2 atmos...

Scheme 56: Electrochemical diastereoselective α-alkylation of pyrrolidines attached with phosphorus-derived ch...

Scheme 57: Electrogenerated cyanomethyl anion-induced synthesis of chiral cis-β-lactams from amides bearing ch...

Scheme 58: Diastereoselective anodic oxidation followed by intramolecular cyclization of ω-hydroxyl amides bea...

Scheme 59: Electrochemical deprotonation of Ni(II) glycinate containing (S)-BPB as a chiral auxiliary: diaster...

Scheme 60: Enantioselective electroreductive coupling of diaryl ketones with α,β-unsaturated carbonyl compound...

Scheme 61: Asymmetric total synthesis of ropivacaine and its analogues using a electroorganic reaction as a ke...

Scheme 62: Asymmetric total synthesis of (−)-crispine A and its natural enantiomer via anodic cyanation of tet...

Scheme 63: Asymmetric oxidative electrodimerization of cinnamic acid derivatives as key step for the synthesis...
N-(1-Phenylethyl)aziridine-2-carboxylate esters in the synthesis of biologically relevant compounds
- Iwona E. Głowacka,
- Aleksandra Trocha,
- Andrzej E. Wróblewski and
- Dorota G. Piotrowska
Beilstein J. Org. Chem. 2019, 15, 1722–1757, doi:10.3762/bjoc.15.168

- of chemical and configurational stability was achieved by introducing N,N-dibenzylserinals 4 [10]. Another important strategy of asymmetric synthesis relies on chiral auxiliaries, i.e., a specially selected homochiral part of a starting material governing the stereoselectivity of subsequent reactions

Graphical Abstract

Figure 1: Examples of three-carbon chirons.

Figure 2: Structures of derivatives of N-(1-phenylethyl)aziridine-2-carboxylic acid 5–8.

Figure 3: Synthetic equivalency of aziridine aldehydes 6.

Scheme 1: Synthesis of N-(1-phenylethyl)aziridine-2-carboxylates 5. Reagents and conditions: a) TEA, toluene,...

Scheme 2: Absolute configuration at C2 in (2S,1'S)-5a. Reagents and conditions: a) 20% HClO4, 80 °C, 30 h the...

Scheme 3: Major synthetic strategies for a 2-ketoaziridine scaffold [R* = (R)- or (S)-1-phenylethyl; R′ = Alk...

Scheme 4: Synthesis of cyanide (2S,1'S)-13. Reagents and conditions: a) NH3, EtOH/H2O, rt, 72 h; b) Ph3P, CCl4...

Scheme 5: Synthesis of key intermediates (R)-16 and (R)-17 for (R,R)-formoterol (14) and (R)-tamsulosin (15)....

Scheme 6: Synthesis of mitotic kinesin inhibitors (2R/S,1'R)-23. Reagents and conditions: a) H2, Pd(OH)2, EtO...

Scheme 7: Synthesis of (R)-mexiletine ((R)-24). Reagents and conditions: a) TsCl, TEA, DMAP, CH2Cl2, rt, 1 h;...

Scheme 8: Synthesis of (−)-cathinone ((S)-27). Reagents and conditions: a) PhMgBr, ether, 0 °C; b) H2, 10% Pd...

Scheme 9: Synthesis of N-Boc-norpseudoephedrine ((1S,2S)-(+)-29) and N-Boc-norephedrine ((1R,2S)-29). Reagent...

Scheme 10: Synthesis of (−)-ephedrine ((1R,2S)-31). Reagents and conditions: a) TfOMe, MeCN then NaBH3CN, rt; ...

Scheme 11: Synthesis of xestoaminol C ((2S,3R)-35), 3-epi-xestoaminol C ((2S,3S)-35) and N-Boc-spisulosine ((2S...

Scheme 12: Synthesis of ʟ-tryptophanol ((S)-41). Reagents and conditions: a) CDI, MeCN, rt, 1 h then TMSI, MeC...

Scheme 13: Synthesis of ʟ-homophenylalaninol ((S)-42). Reagents and conditions: a) NaH, THF, 0 °C to −78 °C, 1...

Scheme 14: Synthesis of ᴅ-homo(4-octylphenyl)alaninol ((R)-47) and a sphingolipid analogue (R)-48. Reagents an...

Scheme 15: Synthesis of florfenicol ((1R,2S)-49). Reagents and conditions: a) (S)-1-phenylethylamine, TEA, MeO...

Scheme 16: Synthesis of natural tyroscherin ((2S,3R,6E,8R,10R)-55). Reagents and conditions: a) I(CH2)3OTIPS, t...

Scheme 17: Syntheses of (−)-hygrine (S)-61, (−)-hygroline (2S,2'S)-62 and (−)-pseudohygroline (2S,2'R)-62. Rea...

Scheme 18: Synthesis of pyrrolidine (3S,3'R)-68, a fragment of the fluoroquinolone antibiotic PF-00951966. Rea...

Scheme 19: Synthesis of sphingolipid analogues (R)-76. Reagents and conditions: a) BnBr, Mg, THF, reflux, 6 h;...

Scheme 20: Synthesis of ᴅ-threo-PDMP (1R,2R)-81. Reagents and conditions: a) TMSCl, NaI, MeCN, rt, 1 h 50 min,...

Scheme 21: Synthesis of the sphingolipid analogue SG-14 (2S,3S)-84. Reagents and conditions: a) LiAlH4, THF, 0...

Scheme 22: Synthesis of the sphingolipid analogue SG-12 (2S,3R)-88. Reagents and conditions: a) 1-(bromomethyl...

Scheme 23: Synthesis of sphingosine-1-phosphate analogues DS-SG-44 and DS-SG-45 (2S,3R)-89a and (2S,3R)-89a. R...

Scheme 24: Synthesis of N-Boc-safingol ((2S,3S)-95) and N-Boc-ᴅ-erythro-sphinganine ((2S,3R)-95). Reagents and...

Scheme 25: Synthesis of ceramide analogues (2S,3R)-96. Reagents and conditions: a) NaBH4, ZnCl2, MeOH, −78 °C,...

Scheme 26: Synthesis of orthogonally protected serinols, (S)-101 and (R)-102. Reagents and conditions: a) BnBr...

Scheme 27: Synthesis of N-acetyl-3-phenylserinol ((1R,2R)-105). Reagents and conditions: a) AcOH, CH2Cl2, refl...

Scheme 28: Synthesis of (S)-linezolid (S)-107. Reagents and conditions: a) LiAlH4, THF, 0 °C to reflux; b) Boc2...

Scheme 29: Synthesis of (2S,3S,4R)-2-aminooctadecane-1,3,4-triol (ᴅ-ribo-phytosphingosine) (2S,3S,4R)-110. Rea...

Scheme 30: Syntheses of ᴅ-phenylalanine (R)-116. Reagents and conditions: a) AcOH, CH2Cl2, reflux, 4 h; b) MsC...

Scheme 31: Synthesis of N-Boc-ᴅ-3,3-diphenylalanine ((R)-122). Reagents and conditions: a) PhMgBr, THF, −78 °C...

Scheme 32: Synthesis of ethyl N,N’-di-Boc-ʟ-2,3-diaminopropanoate ((S)-125). Reagents and conditions: a) NaN3,...

Scheme 33: Synthesis of the bicyclic amino acid (S)-(+)-127. Reagents and conditions: a) BF3·OEt2, THF, 60 °C,...

Scheme 34: Synthesis of lacosamide, (R)-2-acetamido-N-benzyl-3-methoxypropanamide (R)-130. Reagents and condit...

Scheme 35: Synthesis of N-Boc-norfuranomycin ((2S,2'R)-133). Reagents and conditions: a) H2C=CHCH2I, NaH, THF,...

Scheme 36: Synthesis of MeBmt (2S,3R,4R,6E)-139. Reagents and conditions: a) diisopropyl (S,S)-tartrate (E)-cr...

Scheme 37: Synthesis of (+)-polyoxamic acid (2S,3S,4S)-144. Reagents and conditions: a) AD-mix-α, MeSO2NH2, t-...

Scheme 38: Synthesis of the protected 3-hydroxy-ʟ-glutamic acid (2S,3R)-148. Reagents and conditions: a) LiHMD...

Scheme 39: Synthesis of (+)-isoserine (R)-152. Reagents and conditions: a) AcCl, MeCN, rt, 0.5 h then Na2CO3, ...

Scheme 40: Synthesis of (3R,4S)-N3-Boc-3,4-diaminopentanoic acid (3R,4S)-155. Reagents and conditions: a) Ph3P...

Scheme 41: Synthesis of methyl (2S,3S,4S)-4-(dimethylamino)-2,3-dihydroxy-5-methoxypentanoate (2S,3S,4S)-159. ...

Scheme 42: Syntheses of methyl (3S,4S) 4,5-di-N-Boc-amino-3-hydroxypentanoate ((3S,4S)-164), methyl (3S,4S)-4-N...

Scheme 43: Syntheses of (3R,5S)-5-(aminomethyl)-3-(4-methoxyphenyl)dihydrofuran-2(3H)-one ((3R,5S)-168). Reage...

Scheme 44: Syntheses of a series of imidazolin-2-one dipeptides 175–177 (for R' and R'' see text). Reagents an...

Scheme 45: Syntheses of (2S,3S)-N-Boc-3-hydroxy-2-hydroxymethylpyrrolidine ((2S,3S)-179). Reagents and conditi...

Scheme 46: Syntheses of enantiomers of 1,4-dideoxy-1,4-imino-ʟ- and -ᴅ-lyxitols (2S,3R,4S)-182 and (2R,3S,4R)-...

Scheme 47: Synthesis of 1,4-dideoxy-1,4-imino-ʟ-ribitol (2S,3S,4R)-182. Reagents and conditions: a) AcOH, CH2Cl...

Scheme 48: Syntheses of 1,4-dideoxy-1,4-imino-ᴅ-arabinitol (2R,3R,4R)-182 and 1,4-dideoxy-1,4-imino-ᴅ-xylitol ...

Scheme 49: Syntheses of natural 2,5-imino-2,5,6-trideoxy-ʟ-gulo-heptitol ((2S,3R,4R,5R)-184) and its C4 epimer...

Scheme 50: Syntheses of (−)-dihydropinidine ((2S,6R)-187a) (R = C3H7) and (2S,6R)-isosolenopsins (2S,6R)-187b ...

Scheme 51: Syntheses of (+)-deoxocassine ((2S,3S,6R)-190a, R = C12H25) and (+)-spectaline ((2S,3S,6R)-190b, R ...

Scheme 52: Synthesis of (−)-microgrewiapine A ((2S,3R,6S)-194a) and (+)-microcosamine A ((2S,3R,6S)-194b). Rea...

Scheme 53: Syntheses of ʟ-1-deoxynojirimycin ((2S,3S,4S,5R)-200), ʟ-1-deoxymannojirimycin ((2S,3S,4S,5S)-200) ...

Scheme 54: Syntheses of 1-deoxy-ᴅ-galacto-homonojirimycin (2R,3S,4R,5S)-211. Reagents and conditions: a) MeONH...

Scheme 55: Syntheses of 7a-epi-hyacinthacine A1 (1S,2R,3R,7aS)-220. Reagents and conditions: a) TfOTBDMS, 2,6-...

Scheme 56: Syntheses of 8-deoxyhyacinthacine A1 ((1S,2R,3R,7aR)-221). Reagents and conditions: a) H2, Pd/C, PT...

Scheme 57: Syntheses of (+)-lentiginosine ((1S,2S,8aS)-227). Reagents and conditions: a) (EtO)2P(O)CH2COOEt, L...

Scheme 58: Syntheses of 8-epi-swainsonine (1S,2R,8S,8aR)-231. Reagents and conditions: a) Ph3P=CHCOOMe, MeOH, ...

Scheme 59: Synthesis of a protected vinylpiperidine (2S,3R)-237, a key intermediate in the synthesis of (−)-sw...

Scheme 60: Synthesis of a modified carbapenem 245. Reagents and conditions: a) AcOEt, LiHMDS, THF, −78 °C, 1.5...
Dirhodium(II)-catalyzed [3 + 2] cycloaddition of N-arylaminocyclopropane with alkyne derivatives
- Wentong Liu,
- Yi Kuang,
- Zhifan Wang,
- Jin Zhu and
- Yuanhua Wang
Beilstein J. Org. Chem. 2019, 15, 542–550, doi:10.3762/bjoc.15.48
- method has potential synthetic practicality by providing a convenient way to synthesize trans-cyclic β-amino acid derivatives. Further application of this method with other cycloaddition partners and asymmetric synthesis of the chiral ring with the help of chiral auxiliaries are currently underway

Graphical Abstract

Scheme 1: Applications of N-arylaminocyclopropanes.

Scheme 2: Synthesis of trans-ethyl 2-aminocyclopentanecarboxylate.

Scheme 3: Proposed mechanism.
Synthesis of nonracemic hydroxyglutamic acids
- Dorota G. Piotrowska,
- Iwona E. Głowacka,
- Andrzej E. Wróblewski and
- Liwia Lubowiecka
Beilstein J. Org. Chem. 2019, 15, 236–255, doi:10.3762/bjoc.15.22

- ) also appeared attractive providing two or three predefined stereogenic centers. In more sophisticated approaches application of chiral auxiliaries allowed to generate vicinal or 1,3-aminoalcohol units of the required stereochemistries. Currently available synthetic methodologies towards hydroxyglutamic

Graphical Abstract

Figure 1: Structure of L-glutamic acid.

Figure 2: 3-Hydroxy- (2), 4-hydroxy- (3) and 3,4-dihydroxyglutamic acids (4).

Figure 3: Enantiomers of 3-hydroxyglutamic acid (2).

Scheme 1: Synthesis of (2S,3R)-2 from (R)-Garner's aldehyde. Reagents and conditions: a) MeOCH=CH–CH(OTMS)=CH2...

Scheme 2: Synthesis of (2S,3R)-2 and (2S,3S)-2 from (R)-Garner’s aldehyde. Reagents and conditions: a) H2C=CH...

Scheme 3: Two-carbon homologation of the protected L-serine. Reagents and conditions: a) Fmoc-succinimide, Na2...

Scheme 4: Synthesis of di-tert-butyl ester of (2R,3S)-2 from L-serine. Reagents and conditions: a) PhSO2Cl, K2...

Scheme 5: Synthesis of (2R,3S)-2 from O-benzyl-L-serine. Reagents and conditions: a) (CF3CH2O)2P(O)CH2COOMe, ...

Scheme 6: Synthesis of (2S,3R)-2 employing a one-pot cis-olefination–conjugate addition sequence. Reagents an...

Scheme 7: Synthesis of the orthogonally protected (2S,3R)-2 from a chiral aziridine. Reagents and conditions:...

Scheme 8: Synthesis of N-Boc-protected (2S,3R)-2 from D-phenylglycine. Reagents and conditions: a) BnMgCl, et...

Scheme 9: Synthesis of (2S,3R)-2 employing ketopinic acid as chiral auxiliary. Reagents and conditions: a) Br2...

Scheme 10: Synthesis of dimethyl ester of (2S,3R)-2 employing (1S)-2-exo-methoxyethoxyapocamphane-1-carboxylic...

Scheme 11: Synthesis of N-Boc-protected dimethyl ester of (2S,3R)-2 from (S)-N-(1-phenylethyl)thioacetamide. R...

Scheme 12: Synthesis of N-Boc-protected dimethyl ester of (2S,3R)-2 via Sharpless epoxidation. Reagents and co...

Scheme 13: Synthesis of (2S,3S)-2 from the imide 51. Reagents and conditions: a) NaBH4, MeOH/CH2Cl2; b) Ac2O, ...

Scheme 14: Synthesis of (2R,3S)-2 and (2S,3S)-2 from the acetolactam 55 (PMB = p-methoxybenzyl). Reagents and ...

Scheme 15: Synthesis of (2S,3R)-2 from D-glucose. Reagents and conditions: a) NaClO2, 30% H2O2, NaH2PO4, MeCN;...

Figure 4: Enantiomers of 3-hydroxyglutamic acid (3).

Scheme 16: Synthesis of (4S)-4-hydroxy-L-glutamic acid [(2S,4S)-3] by electrophilic hydroxylation. Reagents an...

Scheme 17: Synthesis of all stereoisomers of 4-hydroxyglutamic acid (3). Reagents and conditions: a) Br2, PBr5...

Scheme 18: Synthesis of the orthogonally protected 4-hydroxyglutamic acid (2S,4S)-73. Reagents and conditions:...

Scheme 19: Synthesis of (2S,4R)-4-acetyloxyglutamic acid as a component of a dipeptide. Reagents and condition...

Scheme 20: Synthesis of N-Boc-protected dimethyl esters of (2S,4R)- and (2S,4S)-3 from (2S,4R)-4-hydroxyprolin...

Scheme 21: Synthesis of orthogonally protected (2S,4S)-3 from (2S,4R)-4-hydroxyproline. Reagents and condition...

Scheme 22: Synthesis of the protected (4R)-4-hydroxy-L-pyroglutamic acid (2S,4R)-87 by electrophilic hydroxyla...

Figure 5: Enantiomers of 3,4-dihydroxy-L-glutamic acid (4).

Scheme 23: Synthesis of (2S,3S,4R)-4 from the epoxypyrrolidinone 88. Reagents and conditions: a) MeOH, THF, KC...

Scheme 24: Synthesis of (2S,3R,4R)-4 from the orthoester 92. Reagents and conditions: a) OsO4, NMO, acetone/wa...

Scheme 25: Synthesis of (2S,3S,4S)-4 from the aziridinolactone 95. Reagents and conditions: a) BnOH, BF3·OEt2,...

Scheme 26: Synthesis of (2S,3S,4R)-4 and (2R,3S,4R)-4 from cyclic imides 106. Reagents and conditions: a) NaBH4...

Scheme 27: Synthesis of (2R,3R,4R)-4 and (2S,3R,4R)-4 from the cyclic meso-imide 110. Reagents and conditions:...

Scheme 28: Synthesis of (2S,3S,4S)-4 from the protected serinal (R)-23. Reagents and conditions: a) Ph3P=CHCOO...

Scheme 29: Synthesis of (2S,3S,4S)-4 from O-benzyl-N-Boc-D-serine. Reagents and conditions: a) ClCOOiBu, TEA, ...

Scheme 30: Synthesis of (2S,3S,4R)-127 by enantioselective conjugate addition and asymmetric dihydroxylation. ...

Figure 6: Structures of selected compounds containing hydroxyglutamic motives (in blue).
Some mechanistic aspects regarding the Suzuki–Miyaura reaction between selected ortho-substituted phenylboronic acids and 3,4,5-tribromo-2,6-dimethylpyridine
- Piotr Pomarański,
- Piotr Roszkowski,
- Jan K. Maurin,
- Armand Budzianowski and
- Zbigniew Czarnocki
Beilstein J. Org. Chem. 2018, 14, 2384–2393, doi:10.3762/bjoc.14.214
- ’-bromopyridinium N-(2’-pyrazinyl)aminides [17]. In 2014, a regiocontrolled polyarylation of pyridine was presented by Doebelin and co-workers [27]. Rotationally restricted biaryls are stereochemically and structurally important elements of a rapidly growing class of catalysts, natural products and chiral
- auxiliaries. Therefore, the atropselective synthesis is an important synthetic approach pursued by many research groups [22][23][24][25][26]. In 2015 the first phosphoric acid-catalysed asymmetric reactions of 2-naphthols with quinone analogues were described, allowing an access to a class of sterically

Graphical Abstract

Figure 1: Structures of stereoisomers of 3,4,5-tris(2-methoxyphenyl)-2,6-dimethylpyridines determined by X-ra...

Figure 2: Graphical representation of kinetic, time-dependent 1H NMR analysis of (syn)-7 (100 °C).

Figure 3: Graphical representation of kinetic, time-dependent 1H NMR analysis of (syn)-10 (120 °C).

Figure 4: HT-NMR (300 MHz, DMSO-d6) spectra of A) (syn)-7. B) (syn)-10. Only the upfield (ca. 3.4–4 ppm) regi...

Figure 5: Summary of the results for coupling with ortho-substituted phenylboronic acid for triaryl products.

Figure 6: Summary of results for coupling with ortho-substituted phenylboronic acid for diaryl products.

Figure 7: Proposed intermediates for the 1,2-addition of 5 with methoxy group. A) Oxidative addition step. B)...

Figure 8: Proposed intermediates for the 1,3-addition with methoxy group. A) Oxidative addition step. B) Tran...

Figure 9: Proposed intermediates for the 1,2-addition with chlorine atom. A) Oxidative addition step. B) Tran...

Figure 10: Proposed intermediates for the 1,3-addition with chlorine atom. A) Oxidative addition step. B) Tran...
Synthesis of spirocyclic scaffolds using hypervalent iodine reagents
- Fateh V. Singh,
- Priyanka B. Kole,
- Saeesh R. Mangaonkar and
- Samata E. Shetgaonkar
Beilstein J. Org. Chem. 2018, 14, 1778–1805, doi:10.3762/bjoc.14.152
- chiral auxiliaries 129a or 129b under similar reaction conditions mentioned in Scheme 48. Furthermore, the synthesized spiroketal 159 (R2 = iPr; R4 = SiMe3) was used as synthetic intermediate for enantioselective synthesis of natural product (−)-biscarvacrol [8] (Scheme 59). Additionally, Parra and

Graphical Abstract

Figure 1: The structures of biologically active natural and synthetic products having spirocyclic moiety.

Scheme 1: Iodine(III)-mediated spirocyclization of substituted phenols 7 and 11 to 10 and 13, respectively.

Scheme 2: PIDA-mediated spirolactonization of N-protected tyrosine 14 to spirolactone 16.

Figure 2: The structures of polymer-supported iodine(III) reagents 17a and 17b.

Scheme 3: Spirolactonization of substrates 14 to spirolactones 16 using polymer-supported reagents 17a and 17b...

Scheme 4: PIDA-mediated spirolactonization of 1-(p-hydroxyaryl)cyclobutanols 18 to spirolactones 19.

Scheme 5: Iodine(III)-mediated spirocyclization of aryl alkynes 24 to spirolactones 26 by the reaction with b...

Scheme 6: Bridged iodine(III)-mediated spirocyclization of phenols 27 to spirodienones 29.

Scheme 7: Iodine(III)-mediated spirocyclization of arnottin I (30) to its spirocyclic analogue arnottin II (32...

Scheme 8: Iodine(III)-catalyzed spirolactonization of p-substituted phenols 27 to spirolactones 29 using iodo...

Scheme 9: Iodine(III)-catalyzed oxylactonization of ketocarboxylic acid 34 to spirolactone 36 using iodobenze...

Scheme 10: Iodine(III)-mediated asymmetric oxidative spirocyclization of naphthyl acids 37 to naphthyl spirola...

Scheme 11: Oxidative cyclization of L-tyrosine 14 to spirocyclic lactone 16 using PIDA (15).

Scheme 12: Oxidative cyclization of oxazoline derivatives 41 to spirolactams 42 using PIDA (15).

Scheme 13: Oxidative cyclization of oxazoline 43 to spirolactam 44 using PIDA 15 as oxidant.

Scheme 14: PIFA-mediated spirocyclization of amides 46 to N-spirolactams 47 using PIFA (31) as an electrophile....

Scheme 15: Synthesis of spirolactam 49 from phenolic enamide 48 using PIDA (15).

Scheme 16: Iodine(III)-mediated spirocyclization of alkyl hydroxamates 50 to spirolactams 51 using stoichiomet...

Scheme 17: PIFA-mediated cyclization of substrate 52 to spirocyclic product 54.

Scheme 18: Synthesis of spiro β-lactams 56 by oxidative coupling reaction of p-substituted phenols 55 using PI...

Scheme 19: Iodine(III)-mediated spirocyclization of para-substituted amide 58 to spirolactam 59 by the reactio...

Scheme 20: Iodine(III)-mediated synthesis of spirolactams 61 from anilide derivatives 60.

Scheme 21: PIFA-mediated oxidative cyclization of anilide 60 to bis-spirobisoxindole 61.

Scheme 22: PIDA-mediated spirocyclization of phenylacetamides 65 to spirocyclic lactams 66.

Scheme 23: Oxidative dearomatization of arylamines 67 with PIFA (31) to give dieniminium salts 68.

Scheme 24: PIFA-mediated oxidative spirocarbocyclization of 4-methoxybenzamide 69 with diphenylacetylene (70) ...

Scheme 25: Synthesis of spiroxyindole 75 using I2O5/TBHP oxidative system.

Scheme 26: Iodine(III)-catalyzed spirolactonization of functionalized amides 76 to spirolactones 77 using iodo...

Scheme 27: Intramolecular cyclization of alkenes 78 to spirolactams 80 using Pd(II) 79 and PIDA (15) as the ox...

Scheme 28: Iodine(III)-catalyzed spiroaminocyclization of amides 76 to spirolactam 77 using bis(iodoarene) 81 ...

Scheme 29: Iodine(III)-catalyzed spirolactonization of N-phenyl benzamides 82 to spirolactams 83 using iodoben...

Scheme 30: Iodine(III)-mediated asymmetric oxidative spirocyclization of phenols 84 to spirolactams 86 using c...

Scheme 31: Iodine(III)-catalyzed asymmetric oxidative spirocyclization of N-aryl naphthamides 87 to spirocycli...

Scheme 32: Cyclization of p-substituted phenolic compound 89 to spirolactam 90 using PIDA (15) in TFE.

Scheme 33: Iodine(III)-mediated synthesis of spirocyclic compound 93 from substrates 92 using PIDA (15) as an ...

Scheme 34: Iodine(III)-mediated spirocyclization of p-substituted phenol 48 to spirocyclic compound 49 using P...

Scheme 35: Bridged iodine(III)-mediated spirocyclization of O-silylated phenolic compound 96 in the synthesis ...

Scheme 36: PIFA-mediated approach for the spirocyclization of ortho-substituted phenols 98 to aza-spirocarbocy...

Scheme 37: Oxidative cyclization of para-substituted phenols 102 to spirocarbocyclic compounds 104 using Koser...

Scheme 38: Iodine(III)-mediated spirocyclization of aryl alkynes 105 to spirocarbocyclic compound 106 by the r...

Scheme 39: Iodine(III)-mediated spirocarbocyclization of ortho-substituted phenols 107 to spirocarbocyclic com...

Scheme 40: PIFA-mediated oxidative cyclization of substrates 110 to spirocarbocyclic compounds 111.

Scheme 41: Iodine(III)-mediated cyclization of substrate 113 to spirocyclic compound 114.

Scheme 42: Iodine(III)-mediated spirocyclization of phenolic substrate 116 to the spirocarbocyclic natural pro...

Scheme 43: Iodine(III)-catalyzed spirocyclization of phenols 117 to spirocarbocyclic products 119 using iodoar...

Scheme 44: PIFA-mediated spirocyclization of 110 to spirocyclic compound 111 using PIFA (31) as electrophile.

Scheme 45: PIDA-mediated spirocyclization of phenolic sulfonamide 122 to spiroketones 123.

Scheme 46: Iodine(III)-mediated oxidative spirocyclization of 2-naphthol derivatives 124 to spiropyrrolidines ...

Scheme 47: PIDA-mediated oxidative spirocyclization of m-substituted phenols 126 to tricyclic spiroketals 127.

Figure 3: The structures of chiral organoiodine(III) catalysts 129a and 129b.

Scheme 48: Iodine(III)-catalyzed oxidative spirocyclization of substituted phenols 128 to spirocyclic ketals 1...

Scheme 49: Oxidative spirocyclization of para-substituted phenol 131 to spirodienone 133 using polymer support...

Scheme 50: Oxidative cyclization of bis-hydroxynaphthyl ether 135 to spiroketal 136 using PIDA (15) as an elec...

Scheme 51: Oxidative spirocyclization of phenolic compound 139 to spirodienone 140 using polymer-supported PID...

Scheme 52: PIFA-mediated oxidative cyclization of catechol derived substrate 142 to spirocyclic product 143.

Scheme 53: Oxidative spirocyclization of p-substituted phenolic substrate 145 to aculeatin A (146a) and aculea...

Scheme 54: Oxidative spirocyclization of p-substituted phenolic substrate 147 to aculeatin A (146a) and aculea...

Scheme 55: Oxidative spirocyclization of p-substituted phenolic substrate 148 to aculeatin D (149) using elect...

Scheme 56: Cyclization of phenolic substrate 131 to spirocyclic product 133 using polymer-supported PIFA 150.

Scheme 57: Iodine(III)-mediated oxidative intermolecular spirocyclization of 7-methoxy-α-naphthol (152) to spi...

Scheme 58: Oxidative cyclization of phenols 155 to spiro-ketals 156 using electrophilic species PIDA (15).

Scheme 59: Iodine(III)-catalyzed oxidative spirocyclization of ortho-substituted phenols 158 to spirocyclic ke...
Diastereoselective auxiliary- and catalyst-controlled intramolecular aza-Michael reaction for the elaboration of enantioenriched 3-substituted isoindolinones. Application to the synthesis of a new pazinaclone analogue
- Romain Sallio,
- Stéphane Lebrun,
- Frédéric Capet,
- Francine Agbossou-Niedercorn,
- Christophe Michon and
- Eric Deniau
Beilstein J. Org. Chem. 2018, 14, 593–602, doi:10.3762/bjoc.14.46
- incorporate α-methylbenzylamine-type chiral auxiliaries, which have been extensively used by Davies et al. to gain access to a wide range of chiral N-heterocycles via intermolecular aza-Michael reactions [34][47][48][49][50][51]. The starting unsaturated benzoic acids 14a–e and 15 were readily prepared via a
- previously observed in other Michael-additions involving chiral auxiliaries on the nucleophile and on the Michael acceptor [62]. As for (S)-6a, catalyst 18c bearing a bulky tert-butyl group at the benzyl para-position gave the best results in term of stereoselectivity (80% de, Table 2, entry 7). However, the

Graphical Abstract

Figure 1: Examples of synthetic pharmacologically active chiral 3-substituted isoindolinones.

Scheme 1: Retrosynthetic analysis of NH free chiral 3-substituted isoindolinones (3S)-1 and (3S)-2.

Scheme 2: Synthesis of parent benzamides 6–8.

Figure 2: Esters 12a–e, 13 prepared, isolated yield.

Figure 3: Benzamides 6a–d, 7a–e, 8 prepared, isolated yield.

Figure 4: Phase transfer catalysts (PTC) used in this study.

Scheme 3: Synthesis of isoindolinones 3a–d, 4a–e, 5; isolated yield, de by HPLC and 1H NMR. aAfter flash chro...

Scheme 4: Removal of the chiral auxiliary. Synthesis of isoindolinones 1a–c, 1e, 2; isolated yield, ee by HPL...

Figure 5: ORTEP plot of isoindolinone (2R,3S)-3a (CCDC 1590565) [68].

Scheme 5: Synthesis of pazinaclone analogue (3S)-27.
Investigations towards the stereoselective organocatalyzed Michael addition of dimethyl malonate to a racemic nitroalkene: possible route to the 4-methylpregabalin core structure
- Denisa Vargová,
- Rastislav Baran and
- Radovan Šebesta
Beilstein J. Org. Chem. 2018, 14, 553–559, doi:10.3762/bjoc.14.42
- amino acids have also been investigated as treatments for ocular disorders [7]. However, the syntheses of this type of compounds were long and relied on the use of Evans chiral auxiliaries or chiral starting materials. Various GABA derivatives were synthesized using asymmetric organocatalysis

Graphical Abstract

Figure 1: Structures of pregabalin and methylpregabalin.

Scheme 1: Synthesis of the nitroalkene 6.

Scheme 2: Catalyst screening in the Michael addition of dimethyl malonate to nitroalkene 6.

Scheme 3: Synthesis of catalysts (Sa,R,R)-C8 and (Sa,S,S)-C8.

Figure 2: Transition state models for the reaction of (R)-6 with dimethyl malonate using catalyst C7 (M06-2X/...

Scheme 4: Synthesis of 4-methylpregabalin (1).
A mechanochemical approach to access the proline–proline diketopiperazine framework
- Nicolas Pétry,
- Hafid Benakki,
- Eric Clot,
- Pascal Retailleau,
- Farhate Guenoun,
- Fatima Asserar,
- Chakib Sekkat,
- Thomas-Xavier Métro,
- Jean Martinez and
- Frédéric Lamaty
Beilstein J. Org. Chem. 2017, 13, 2169–2178, doi:10.3762/bjoc.13.217
- chemists have used DKPs extensively as a synthetic platform, easily synthesized and stereochemically controlled, for the preparation of small bioactive molecules [4][5]. DKPs have also been considered as chiral auxiliaries in asymmetric synthesis [6]. Furthermore, the rigidity of the DKPs is a unique

Graphical Abstract

Scheme 1: Retrosynthesis of the Pro–Pro DKP framework.

Scheme 2: Coupling with N-hydroxysuccinimide-activated amino acids.

Scheme 3: Synthesis of Pro–Pro DKP.

Scheme 4: Synthesis of substituted Pro–Pro DKP 15a.

Scheme 5: Potential isomers yielded by cyclization of 16.

Figure 1: Optimized geometries for the two conformers presenting interactions with either Ca (16a) or Cb (16b...

Figure 2: Optimized geometries of the extrema located along the pathway for formation of 15a with explicit pa...

Figure 3: Optimized geometries of the extrema located along the pathway for formation of 15b with explicit pa...

Figure 4: Optimized geometries for the transition states associated to alternate position of the methanol mol...

Scheme 6: Synthesis of diketopiperazine 19.
Sulfamide chemistry applied to the functionalization of self-assembled monolayers on gold surfaces
- Loïc Pantaine,
- Vincent Humblot,
- Vincent Coeffard and
- Anne Vallée
Beilstein J. Org. Chem. 2017, 13, 648–658, doi:10.3762/bjoc.13.64

- applications in medicinal chemistry, sulfamide groups have been incorporated in self-assembling molecules [22][23][24][25][26][27], peptides [28], polymers [29], ligands [30], chiral auxiliaries [31][32][33] and in organocatalysts [34][35][36][37]. In light of the importance of the sulfamide functionality, our

Graphical Abstract

Scheme 1: General strategy for surface functionalization based on sulfamide chemistry.

Scheme 2: Synthesis of the reference molecule sulfamide 1.

Figure 1: Contact angles of the gold surface, the 4-ATP SAM, the 4-ATP SAM after reaction with ArSO2NHOSO2Ar ...

Figure 2: (a) IR spectra of sulfamide 1 in bulk (solid state) (bottom) and adsorbed on gold (top). (b) PM-IRR...

Figure 3: High resolution S2p and N1s XPS spectra of the 4-ATP SAM, the 4-ATP SAM after reaction with 4-FC6H4...

Figure 4: High resolution S2p and N1s XPS spectra of the SAM 1 before (top) and after hydrolysis (bottom). Ri...
Diastereoselective anodic hetero- and homo-coupling of menthol-, 8-methylmenthol- and 8-phenylmenthol-2-alkylmalonates
- Matthias C. Letzel,
- Hans J. Schäfer and
- Roland Fröhlich
Beilstein J. Org. Chem. 2017, 13, 33–42, doi:10.3762/bjoc.13.5
- Matthias C. Letzel Hans J. Schafer Roland Frohlich Organisch-Chemisches Institut der Westfälischen Wilhelms- Universität, Corrensstraße 40, D-48149 Münster, Germany 10.3762/bjoc.13.5 Abstract Diastereoselective radical coupling was achieved with chiral auxiliaries. The radicals were generated by
- reason, we were interested in exploring the structural influence of chiral auxiliaries (Figure 1) and the carboxylic acid on the diastereoselectivity in the anodic coupling of carboxylic acids. We report here on the diastereoselectivity found in the anodic hetero- and homo-coupling of menthol- (1)-, 8
- so far successful reagents LiAlH4 and DIBAL. Therefore, in the future bulky chiral auxiliaries must be developed that can be partially disassembled before their cleavage and slim cleavage reagents have to be found and explored. Conclusion We developed an efficient procedure for the synthesis of

Graphical Abstract

Figure 1: Menthol auxiliaries 1–4 used in the following anodic coupling reactions.

Scheme 1: Synthesis of carboxylic acids 13a/b–18a/b.

Scheme 2: (a) Preparation of benzyl 2-isopropylmalonate (5) and (b) preparation of benzyl 2-tert-butylmalonat...

Scheme 3: Coelectrolysis (hetero-coupling) of carboxylic acids 13–17 with 3,3-dimethylbutyric acid (20).

Figure 2: Crystal structure of the minor diastereomer 23b.

Figure 3: Cyclic voltammograms of the malonic derivatives 15a/b, 16a/b and 18a/b (scan rate: 500 mA/s, solven...

Scheme 4: Homo-coupling of carboxylic acids 13a/b–16a/b to diesters 26a/b/c–29a/b/c (n.d.: not determined).

Figure 4: Crystal structure of major diastereomer 28a.

Figure 5: Crystal structure of major diastereomer 29a.

Figure 6: Discrimination of diastereomeric faces in the menthol substituted radical A and in the 8-phenylment...

Scheme 5: Reductive cleavage of 30a–c to 8-phenylmenthol (3) and 31a–c.






